I am following up on one unfinished thread in my previous post; a candidate was proposed in which the transition state for [3,3] sigmatropic rearrangement in a semibullvalene might be frozen out to become instead a stable minimum.
The hypothesis was that such a species would be aromatic, a bis-homoaromatic species to be precise, itself an interesting variation on the normal theme of aromaticity. The so-called computed [3,3] vibrational mode emerged as real and not imaginary. But how to go about showing that the resulting species might actually be aromatic? I started with a QTAIM characterisation of bond and ring critical points.
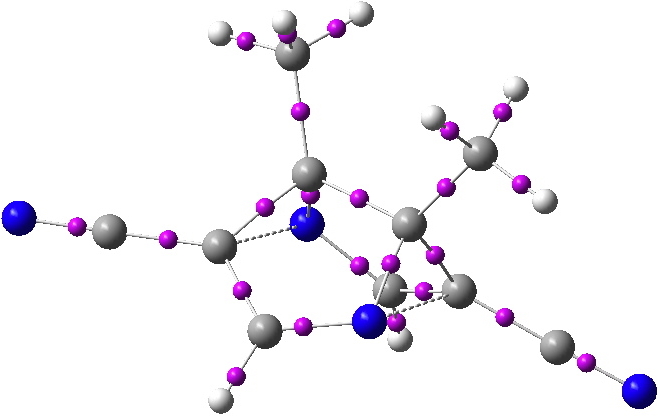
QTAIM analysis of the proposed frozen semibullvalene. Bond critical points in magenta. Click for 3D.
The two homobonds are shown above with dashed bonds. In fact, there are no bond critical points (bcp) located between these two atoms. This of itself need not distress us; the lack of a suitable bcp has been observed previously for other such homo-aromatics[cite]10.1021/jo801022b[/cite], a phenomenon referred to as a no-bond bond. But because the QTAIM does not locate a bond, there is no ring located for the aromatic system either. And in such a heavily non-planar system, the lack of an unambiguous ring centroid means that it is not clear where to place any NICS probe of aromaticity.
What about the ELF technique. For Möbius homoaromaticity[cite]10.1021/ct8001915[/cite] the homo-bond does display a locatable disynaptic ELF basin integrating to around 1-electron. No so here! So no progress yet on estimating the aromaticity.
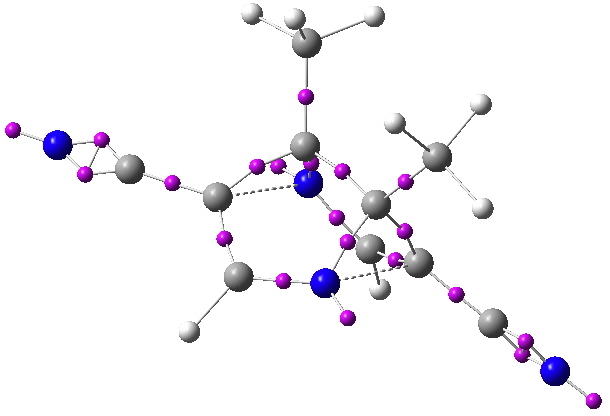
ELF analysis for the frozen [3,3] system. Disynaptic ELF basin centroids in magenta. Click for 3D.
Are there any other computable properties which might be detected experimentally? Well, I noted that the species is C2-symmetric and hence chiral. What about its computed electronic circular dichroism spectrum? These are shown for firstly the frozen system, for which both C-N bonds are 2.13Å in length, and then for a distorted version in which one C-N bond is 1.6Å and the other 2.4Å. The former is more delocalised (as expected for an aromatic ring) and so has λmax in a region which would give the compound a mauve colour. The latter is less delocalised and hence would have an orange-yellow colour, with λmax some 100nm less. The ECD spectra also have different appearances. This would be indeed an interesting way of determining changes in delocalisation, in this case possibly associated with homo-aromaticity.
Tags: pericyclic
[…] shift. So facile that it appears this equilibrium can be frozen out at the transition state if suitable substituents are used. This is a six-electron process, which leads to one of those homologous questions; what happens […]
[…] affect the nature of transition state. For one example where e.g. addition of bulky groups can transform a transition state to a minimum, see here[1] (and there may well be other examples of the reverse transformation of changing a […]