Organic chemistry has some no-go areas, where few molecules dare venture. One of them is described by a concept known as anti-aromaticity. Whereas aromatic molecules are favoured species, their anti-equivalent is avoided. I previously illustrated this (Hückel rule) with cyclopropenium anion. Now I take a look at cyclobutadiene, for which the π-system is said to be iso-electronic (where two electrons in a double bond have replaced the carbanion lone pair).
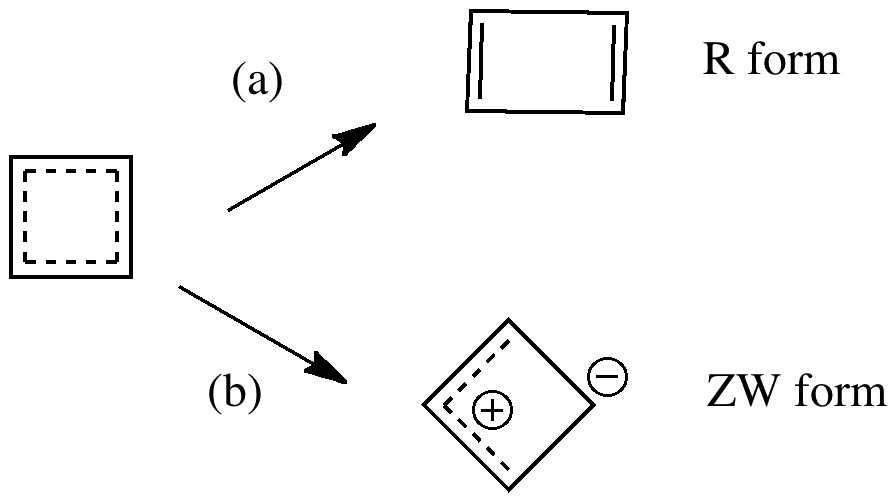
The scheme above starts with a square geometry for the cyclobutadiene. This is strongly anti-aromatic, and the molecule will strive to reduce this by indulging in a geometrical distortion. The conventional distortive mechanism is into an R or rectangular geometry, where two of the C-C bonds get shorter and two longer. The trouble with this mode is that is does not actually prevent the π-π overlaps which made it anti-aromatic in the first place, it just reduces the effect. Thus rectangular cyclobutadiene is still a very very reactive and unstable molecule. So here I suggest another distortion mode, shown above as the ZW, or zwitterionic form. This converts the species into a combination of an allylic carbocation and a secondary carbanion. The latter would be expected to pyramidalize, thus reducing those pesky π-π overlaps. I am unaware of such a ZW-mode ever having been previously explored.
Any student of organic chemistry will be very familiar with how to go about stabilising either a carbocation or a carbanion. We need to do this, since another guiding tenet of organic chemistry is to try to avoid charge separation whenever possible (another almost no-go area). I am going to pull a surprise by evaluating the following model for this post.
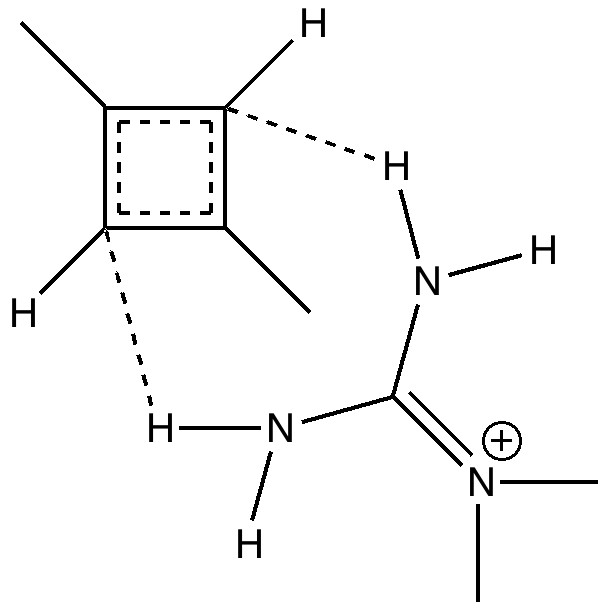
- Firstly, two methyl groups have been placed at the carbocationic centres to stabilise the positive charge. Tertiary carbocations are of course well known to be more stable than secondary ones (I should state that methoxy groups in the same position would stabilise even more, but that is for another post).
- The carbanion could itself be stabilised with an electron withdrawing substituent (say CN) but here I am going to stabilise it with hydrogen bonding to a guanidinium cation. This has just the right shape to form two unusual hydrogen bonds from the N-H to either of the carbanionic lone pairs we might wish to promote (dashed lines above).
- Finally, we are going to simulate this in water as a solvent, in order to stabilize the zwitterion. One zwitterion that DOES form is of course that from the amino acid glycine, but it only forms when placed in water (and life as we know it would not be possible if amino acids did not do this).
| R form | ZW-u form | ZW-s form |
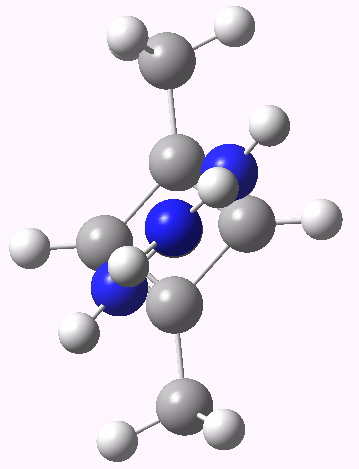 CBD R form. Click for 3D |
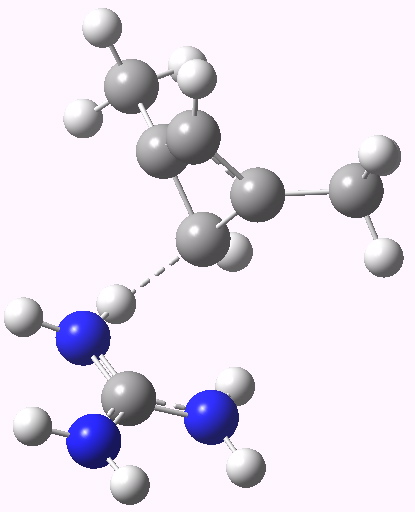 Zwitterionic form. Click for 3D. |
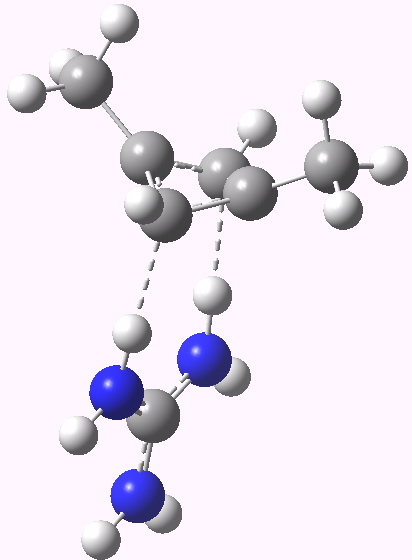 Zwitterionic symmetric form. Click for 3D |
Where have the electrons gone in e.g. the symmetric ZW system? An ELF analysis tells us. The two ELF basins labelled with green arrows contain 1.2 electrons each. The basins corresponding to the 4-ring are labelled with magenta arrows. Put simply, 2.4 electrons have fled the ring, and associated themselves instead with the N-H…C hydrogen bonds. By removing ~2 electrons from an anti-aromatic ring, one converts it into an aromatic one (4n => 4n+2)!
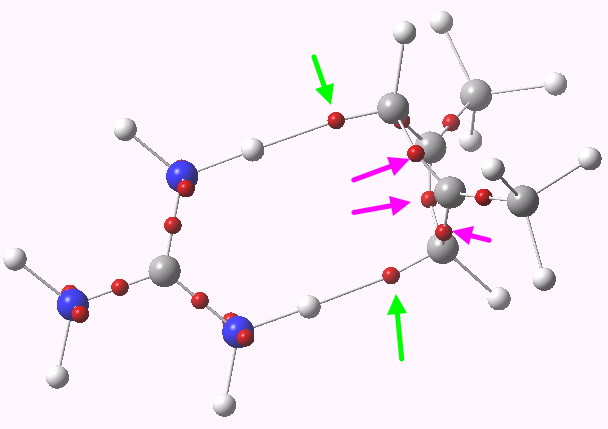
ELF analysis. Click for 3D
Tags: cyclobutadiene, following model for this post, free energy, glycine, Tutorial material, zwitterion
[…] the earlier post on this topic I had explored the possibility of a new isomer of cyclobutadiene, induced by the presence […]
[…] and two πLp electrons from the oxygen. This makes it an anti-aromatic annulene, just like say cyclobutadiene. One aspect of this anti-aromaticity which is not often noted is that the anti-aromaticity makes […]
[…] cyclobutadiene can form a complex with the guanidinium cation in which the anti-aromaticity is reduced by the formation of strong […]