Unusual bonds are always intriguing, and the Xe-Xe bond is no exception. It was first reported (10.1002/anie.199702731) for the species Xe2+. Sb4F21– and its length (3.09Å) was claimed as “unsurpassed in length in main group chemistry by any other element -element bond”. Krapp and Frenking then creatively tweaked the bond (in a computer). The counterion was replaced by C60, and the two xenon atoms placed inside! Buckyballs have a fascinating ability to absorb electrons, up to six in fact, from whatever is placed inside the cavity, and so this assembly acts as a rather intriguing ion-pair. So the issue reduces to how many electrons does C60 manage to scavenge from two Xenon atoms, and what is the nature of any resulting bonding formed between these two atoms?
Before taking a look at that question, I note first a previous post in which I speculate upon the ultimate chemical bond, a 5f-φ bond between two uranium atoms inside C60. The U2 atoms have indeed been stripped of the full complement of six electrons, and the resulting U26+ ion is claimed to support a φ bond. But back to Xe. Krapp and Frenking conclude (from NBO charges) that ~1 electron has been stripped out of Xe2, and the resulting (calculated) Xe-Xe bond length is shortened to 2.49Å, which is actually less than calculated for an isolated Xe22+ ion (2.75Å). This is an unusual (and it has to be said hypothetical) example of a compressed (thermodynamically unstable) bond which would certainly “explode” if released from its prison, reclaiming the electron in the process. The system is also of interest given the unusual nature of the (charge shift) bonding in F2, Cl2, Br2 and also what the effect of injecting electrons into an aromatic periphery of carbon atoms would be (adding two electrons nominally subverts an aromatic 4n+2 rule into an antiaromatic 4n one).
I decided to take another look at Xe2@C60 because since the original study of this species in 2007, computational methodology has evolved to (a) allow the effect of dispersion forces to be included and (b) if the outer skeleton does indeed absorb electrons, a (continuum) solvation correction may also influence the properties. This was done as a ωB97XD/cc-pVDZ/SCRF=water calculation, with aug-cc-pVDZ-pp on Xe.
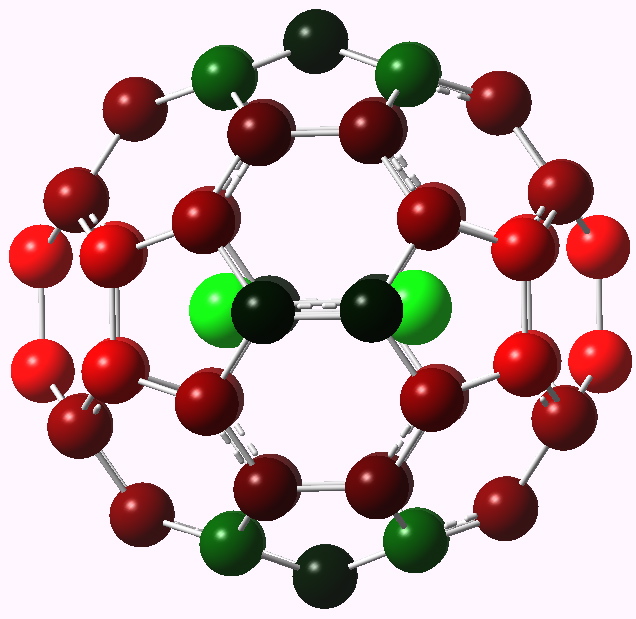
Xe2@C60 showing charge distribution. Click for 3D.
Tags: endohedral complexes, Extreme chemical intimacy, ultimate chemical bond
[…] see here for a good candidate for making a three-layered example (the inside layer is C60, which itself might encapsulate a small molecule). These molecular tools can be created out of quite simple […]