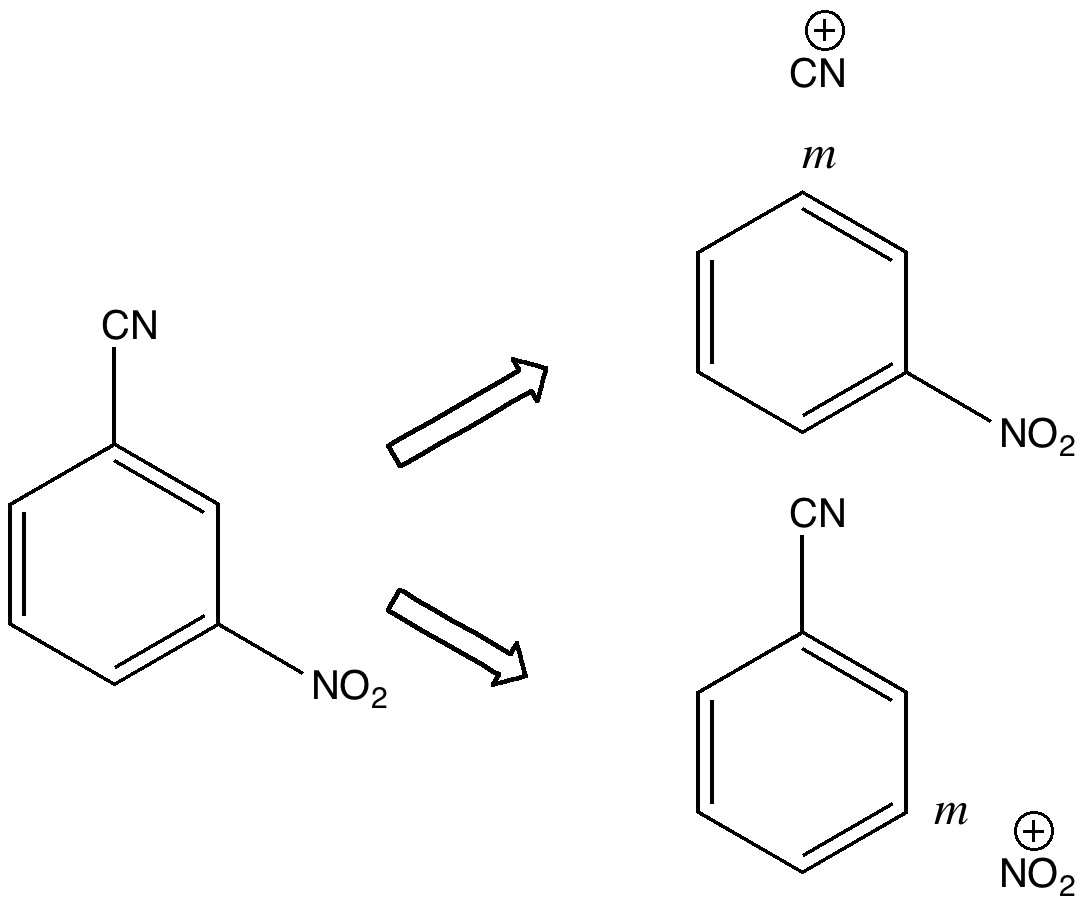
Do you fancy a story going from simplicity to complexity, if not absurdity, in three easy steps? Read on! The following problem appears in one of our (past) examination questions in introductory organic chemistry. From relatively mundane beginnings, one can rapidly find oneself in very unexpected territory.
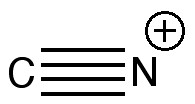
One teaches how to disconnect each group, identifying that both are meta-directing towards electrophiles, and hence asking what an appropriate electrophile might be? The “correct” answer is a nitronium cation (nitric + sulfuric acids) acting upon m-directing benzonitrile. But (sacrilege), why not a “cyonium” cation (CN+) on m-directing nitrobenzene? Well, as a tutor one would normally swat it away on the grounds it has never been previously observed (or that cyanide is always seen as an anion, not a cation). But then one (or a student) asks, why not? How about generating it from e.g. TfOCN. TfO– is a jolly good leaving group, one of the best. Well, this precursor truly appears never to have been made (or even calculated!). By now (if encountered in a tutorial), most chemistry students would be rather bemused. So the process of following one’s nose (more accurately, my nose) continues in the peace and quiet of a blog, where a rather different readership might be bemused (or inflamed).
One might start the same place a student would. How would one represent this diatomic with bonds? How about the above? It has the merit that both atoms are associated with a (shared) octet of electrons, in the form of a quadruple bond. I did show this (briefly) to a colleague, but they recoiled in horror, although it has to be said they were slightly at a loss to actually explain their horror.
Well, time for calculations. How about CCSD/aug-cc-pVTZ (DOI: 10042/to-6261) or B3LYP/aug-cc-pVTZ (DOI: 10042/to-6255). The latter allows a so-called Wiberg bond index to be computed (a reasonably accepted index). This comes out at 3.55, well on the way to being quadruple. An NBO analysis (NBO 5.9) identifies FOUR NBO orbitals with an occupancy of ~2.0, all designated BD (rather than e.g. Lp). What are these NBOs like? (as it turns out, they are almost identical to the MOs for this molecule).
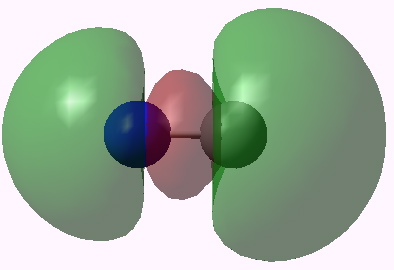 Orbital 7. Click for 3D |
|
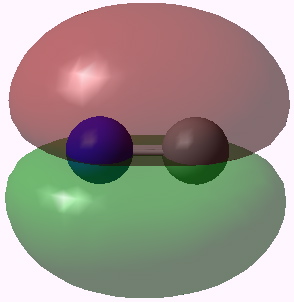 Orbital 6 (π) |
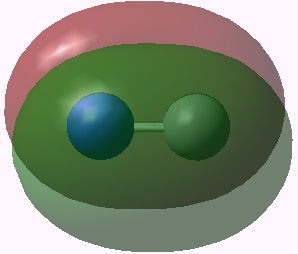 Orbital 5 (π) |
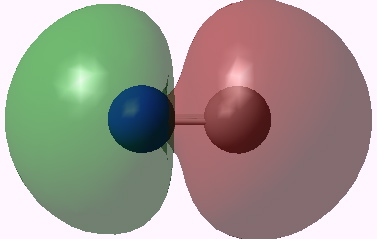 Orbital 4. Click for 3D |
|
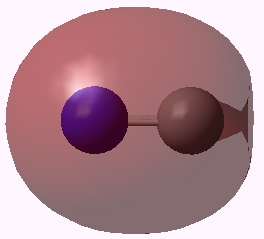 Orbital 3. Click for 3D |
|
Orbitals 5 & 6 are standard π orbitals with no mystery (and 8 & 9, not shown, are the matching π* pair). Orbital 3 results from the overlap of two 2s AOs (but note the curious little toroid at the carbon end). Orbitals 4 and 7 (the LUMO) are the interesting ones. Nominally, the result of overlapping two 2px AOs to give what should be a bonding and antibonding pair, they both appear to be bonding in the C-N region! Perhaps the quadruple bond is not looking quite so unlikely after all (comprising ~double occupancy of orbitals 3-6)!
What about those stalwarts I often use in these blogs, QTAIM and ELF? The former (using the CCSD natural orbitals) has a ρ(r) of 0.346 and a ∇2ρ(r) of +2.01 at the bond-critical point (BCP). The former is certainly a high value, although no calibration exists to compare it to a quadruple bond. The Laplacian has a positive value at this point, possibly an indication of a charge-shift bond (see this and this blog, although more likely due to the adjacency of the bond critical point to the core shell of the carbon atom). ELF (also using natural orbitals) declares the presence of TWO disynaptic basins, with integrations of 5.39e and 2.44 (totalling 7.83e). The basins will each take the form of a torus (see DOI: 10.1021/ct100470g). Hm, perhaps, on reflection, this paragraph might not be entirely suitable for an introductory tutorial to organic chemistry. The density of mumbo-jumbo is rather high!
So starting from a simple retrosynthetic analysis of a simple aromatic molecule, in which the less obvious route is at least considered, one derives a “new” reagent, the cyonium cation CN+. In a effort to analyse its bonding, one concludes that a quadruple bond needs to be taken at least seriously. I would note as a warning that these diatomic species can be really tricky to pin down, and the iso-electronc C2 is a good example of that. But C2 has all sorts of issues, some of which are avoided with CN+. So the last word is hardly written, but not a bad outcome, I venture to suggest, of following one’s nose in a tutorial.
I have appended to this post a 3D exploration of the ELF function, showing the two torus basins referred to above.

ELF function for CN+. Click for 3D
Henry Rzepa, URL:http://www.ch.imperial.ac.uk/rzepa/blog/?p=3065. Accessed: 2011-06-04. (Archived by WebCite® at http://www.webcitation.org/5zBSjBjhM)
Tags: Henry Rzepa, Jahn-Teller, pence, TfO, tutor