The topic of dicarbon, C2, has been discussed here for a few years now. It undoubtedly would be a gas! This aspect of the species came to the fore recently[1] when further experiments on a potential chemical precursor of dicarbon, the zwitterion X(+)-C≡C(-), showed that different variants of X(+), such as not only X=PhI(+), but also e.g. X=dibenzothiophenium(+) appeared to generate a gaseous species, which could be trapped as “C2” in a solvent-free connected flask experiment.
Part of the mystery is that C2 itself is an extremely high energy species, its dimerisation to C4 being around 107 kcal/mol exoenergic in free energy. Now, earlier calculations[2] had revealed that the reaction of the precursor PhI(+)-C≡C(-) with itself can occur on a relatively low energy pathway which avoids the very high energy of C2. The IRC for this reaction is shown below, showing a modest barrier and a very exothermic reaction to the species PhI(+)-CCCC(-) and IPh.
Here I bring your attention to an odd feature on the IRC, in the region of -5. In this region, effectively “free C4” is formed (at an energy some 60 kcal/mol lower than the reactants and 167 kcal/mol lower than two molecules of free C2), but this species is immediately trapped by a PhI to form the final products with a further decrease in energy of ~20 kcal/mol. Suppose however, in a molecular dynamics sense, some proportion of this “C4” could take a different trajectory and free itself at this point, hence escaping being trapped by PhI? This reaction would then generate what again is presumably a gaseous C4.
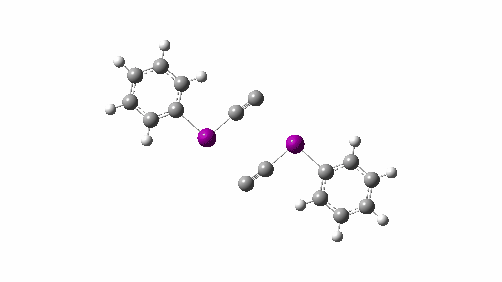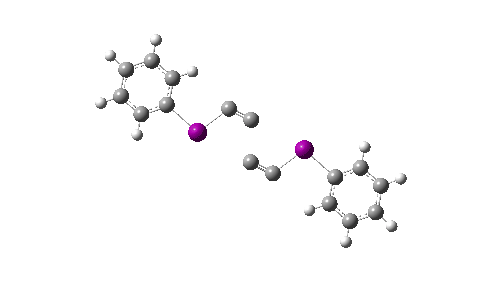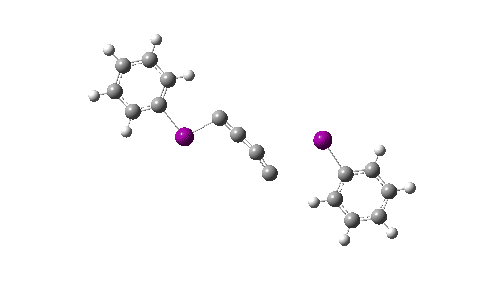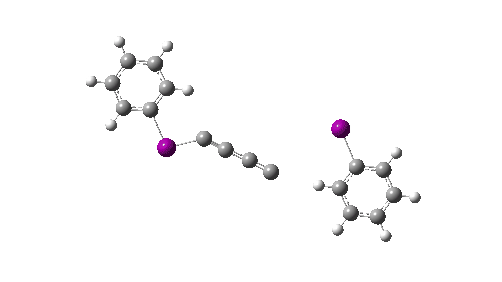
Here I explore what might happen next, to answer the question of whether linear C4 is stable, or will it convert into something else? The scheme below shows some of the possible pathways, leading to the bicyclic form which I have previously discussed extensively in terms of its stabilising aromaticity. Calculations are at the CCSD(T)/Def2-TZVPPD gas phase level, allowing biradicals to form (FAIR Data DOI: 10.14469/hpc/11956).
You can see that C4 is in a modest thermal well, with a free energy barrier to cyclisation of ~22 kcal/mol. So generated at relatively low energies, it might retain its linear structure, whereas at room temperatures or higher, it will probably end up as the bicyclic aromatic species.
The key calculation might be that dimerisation reaction shown above. Would molecular dynamics show that a proportion of the reaction allows the escape of C4? Would that be temperature/pressure dependent? I am not about to try these calculations, but offers of doing so gladly accepted! But that does not necessarily solve the mystery of this reaction, alluded to above.[1] Is the trapped gaseous species C2 itself, C4 in some form, or indeed something else entirely?
This post has DOI: 10.14469/hpc/11959
References
- H.S. Rzepa, M. Arita, K. Miyamoto, and M. Uchiyama, "A combined DFT-predictive and experimental exploration of the sensitivity towards nucleofuge variation in zwitterionic intermediates relating to mechanistic models for unimolecular chemical generation and trapping of free C2 and alternative bimolecular pathways involving no free C2", Physical Chemistry Chemical Physics, vol. 24, pp. 25816-25821, 2022. http://dx.doi.org/10.1039/D2CP01214F
- H.S. Rzepa, "Routes involving no free C2 in a DFT-computed mechanistic model for the reported room-temperature chemical synthesis of C2", Physical Chemistry Chemical Physics, vol. 23, pp. 12630-12636, 2021. http://dx.doi.org/10.1039/D1CP02056K