Previously, a mechanism with a reasonable predicted energy was modelled for the isomerisation of an oxetane carboxylic acid to a lactone by using two further molecules of acid to transfer the proton and in the process encouraging an Sn2 reaction with inversion to open the oxetane ring.
We are now ready to explore variations to this mechanism to see what happens. The first hypothesis is that of replacing two carboxylic acids with one molecule with similar properties, the argument being that bringing two acids together decreases their entropy and hence increases the free energy required for the process. If they come pre-joined, this entropic problem is eliminated and the free energy should reduce. Shown below is a small conjugated molecule with the central OHO motif replaced by NCN instead.
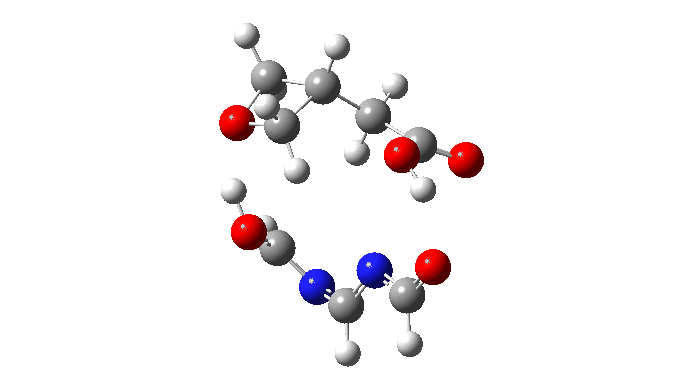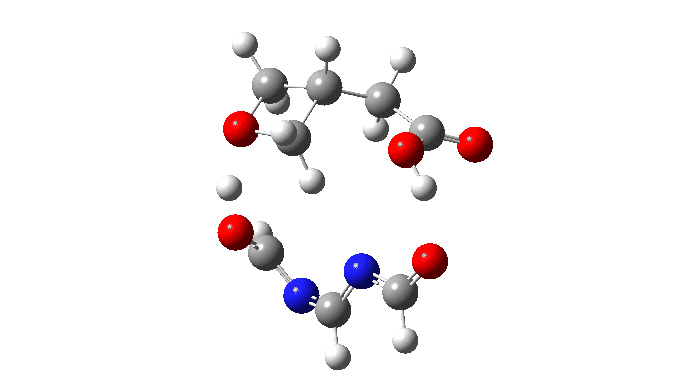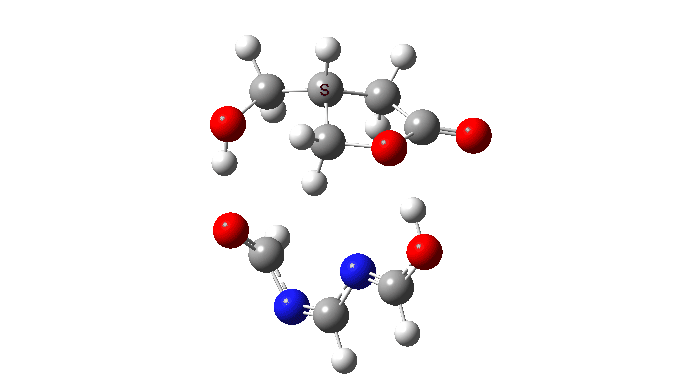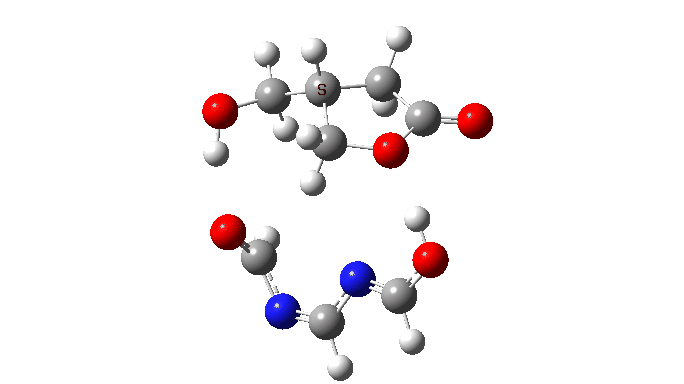
The activation free energy (ωB97XF/Def2-TZVPP, FAIR DOI: 10.14469/hpc/10820) is 18.4 kcal/mol, to be compared to 27.0 when using two carboxylic acids for the transfer. Of course, one would need to optimise the catalyst for many properties, including ease of synthesis, stability, size, isomerism etc, but you get the idea from the procedure here.
The catalyst “designed” here is for proton transfer. One has to wonder whether bespoke catalysts of this type might be useful for any reaction where proton transfer is a vital component!
DOI: 10.14469/hpc/10858 and 10.14469/hpc/10862
Tags: Interesting chemistry