Last May, I wrote an update to the story sparked by the report of the chemical synthesis of C2.[1] This species has a long history of spectroscopic observation in the gas phase, resulting from its generation at high temperatures.[2] The chemical synthesis however was done in solution at ambient or low temperatures, a game-changer as they say. Here I give another update to this unfolding story.
Key to the story is the precursor labelled 11 in the scheme above and the suggestion[1] that it is unimolecular decomposition of 11 that results in C2. A question that had not been posed however was whether 11 itself could participate in any bimolecular reactions and whether these could be lower in free energy than its unimolecular decomposition. That has now been addressed in a recent pre-print, DOI: 10.26434/chemrxiv.13560260.v1[3] Here I will show just one of the possible bimolecular reactions investigated, that of 11 with itself.
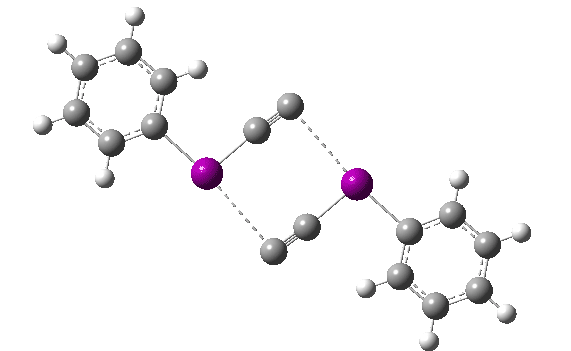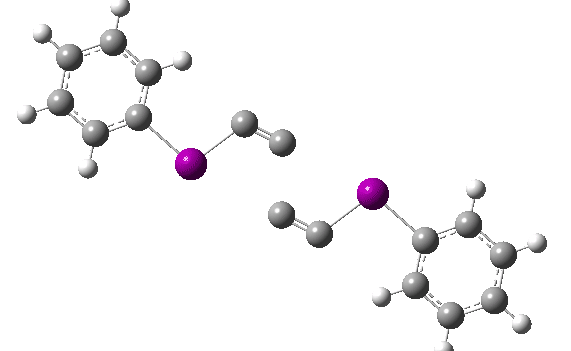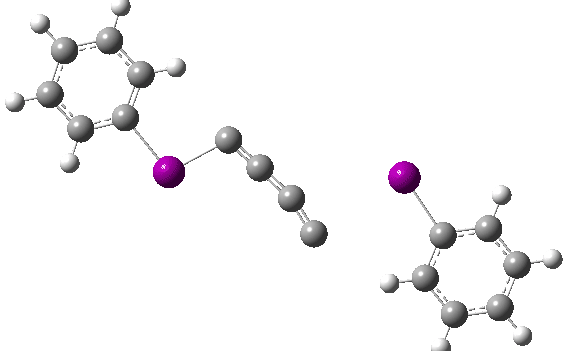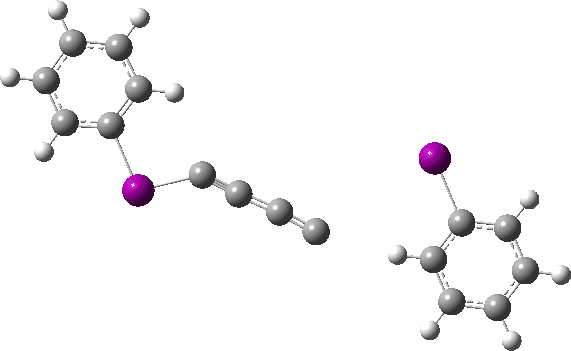
The reaction has a low barrier (ΔG‡ 15.4 kcal/mol for a standard state of 0.044 molar, approximately the concentration the original experiments were conducted for) which means it will be very rapid at room temperatures.♥ The product of this reaction can itself react with more 11 ((ΔG‡ 16.9 kcal/mol) and so on to form polymeric chains or clusters of carbon, eventually resulting in C60 and other forms of carbon. Low energy barriers for a number of other possible bimolecular reactions of 11 with species such as the chemical traps used in the original experiment are also reported,[3] most of which are lower in free energy than that predicted for the unimolecular fragmentation of 11, despite the entropic penalty.
So the enigma is thus: Does species 11 truly fragment to C2, or are the products of this reaction really bimolecular reactions of 11? It does seem as if 11 itself can have a rich and fascinating room temperature chemistry, the scope of which has only started to be explored.
♥The potential energy surface is unusual, in that initially two products are possible, depending on where the C4 unit ends up attached. The potential energy valley only bifurcates into two valleys resulting in the final product at a late stage (~ IRC -5). Put another way, the initial symmetry is C2h, but this breaks/bifurcates into two valleys each leading to different outcomes for the C4 unit. This is very much like the famous potential energy surface for the dimerisation of cyclopentadiene.
References
- K. Miyamoto, S. Narita, Y. Masumoto, T. Hashishin, T. Osawa, M. Kimura, M. Ochiai, and M. Uchiyama, "Room-temperature chemical synthesis of C2", Nature Communications, vol. 11, 2020. http://dx.doi.org/10.1038/s41467-020-16025-x
- T.W. Schmidt, "The Spectroscopy of C2: A Cosmic Beacon", Accounts of Chemical Research, vol. 54, pp. 481-489, 2021. http://dx.doi.org/10.1021/acs.accounts.0c00703
- H. Rzepa, "No Free C2 Is Involved in the DFT-Computed Mechanistic Model for the Reported Room-Temperature Chemical Synthesis of C2.", 2021. http://dx.doi.org/10.26434/chemrxiv.13560260.v1
Tags: Interesting chemistry