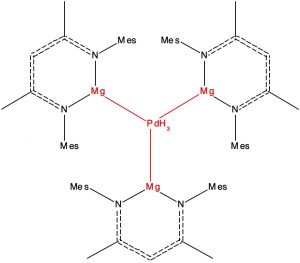
Here is another selection from the Molecules-of-the-Year shortlist published by C&E News, in which hexagonal planar transition metal coordination is identified. This was a mode of metal coordination first mooted more than 100 years ago,[1] but with the first examples only being discovered recently. The C&E News example comprises a central palladium atom surrounded by three hydride and three magnesium atoms, all seven atoms being in the same plane.
As the original article makes clear,[1] the relative orbital simplicity of these early main group based ligands allows the bonding to be better understood, hence itself allowing “additional design principles” to be introduced for transition metal complexes. Here I thought I might extend the scope of this motif by a generalised crystal search for any other hexagonal planar structures to be found in the Cambridge crystal structure database.
A search query can be constructed by defining a plane using the six ligand atoms and then constraining the perpendicular distance between this plane and the transition metal atom at the centre to < 0.1Å. Six angles between adjacent ligands are then themselves constrained to the range 0-70° and the coordination of the central atom can also be constrained to either 6, 7 or 8. All searches are also defined by no disorder and no errors. The search queries can be found at DOI: 10.14469/hpc/6731
I carried out a number of separate searches. The least constrained (any coordination number at the central atom, and any type of attached ligand atom) produced 62 hits, exhibiting a variety of sometimes complex coordination modes. To simplify the search, I separated the searches into specific types. You can view 3D models of any of the molecules below by clicking on the static image.
- The first restricted the transition metal atom to 6-coordinate and for which the ligands all derive from the early main group periods (1A, 2A and H). The sole example (NORLOY[[1], DOI: 10.5517/ccdc.csd.cc2235v7) is the one noted above.
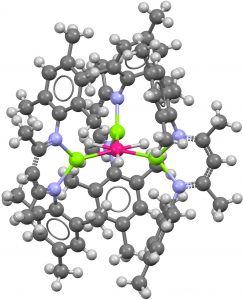
- The next search restricted all the ligands to a transition metal connected to the central atom, along with 6-coordination. Again just one hit (RISQEP[2] dating from 1997 and comprising a central Au atom surrounded by four Au-based ligands and two Fe-based ligands. This type of molecule is a member of a class known as a hexagonal planar metal cluster.
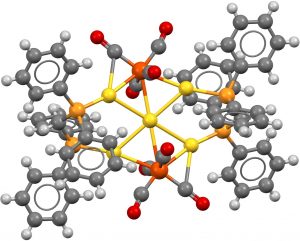
- This search now constrains all six ligands as comprising late main group atoms bonding to the central metal. Two hits, VOVZOV, dating from 1992[3] with a P-based ligand Ni central atom and ZUDWUQ from 1996[4] using As and Ni.
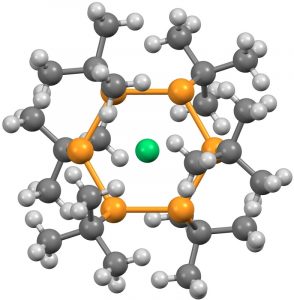
- The next category combines the previous two, with ligands either from the transition series or the late main group series, resulting in four more hits (HACBOF and HACBUL[5] (DOI: 10.5517/ccdc.csd.cc1n26pm and 10.5517/ccdc.csd.cc1n26qn) using a combination of three carbons (as acetylide) and three Ag ligands, with Cu as the central ligand.
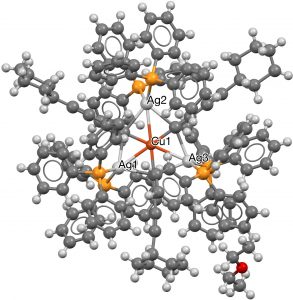
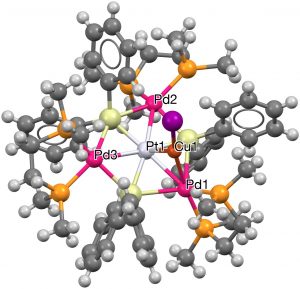
- Next, any ligand is allowed, together with a 7-coordinate central atom. Three examples, including VAPZEU (2016, [6], DOI: 10.5517/ccdc.csd.cc1md045) with three Pd and three Si hexagonal ligands, with an additional 7th Cu surrounding a central Pd.
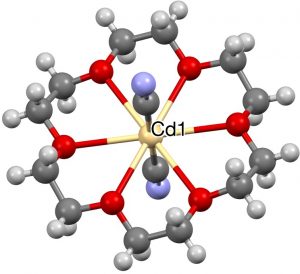
- One example (of eight found) with 8-coordination at central atom (1996, NANPOH[7]), a central Cd, six oxygen ligands and two further cyanide axial ligands.
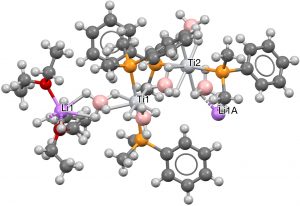
- To finish, a rather wacky polymeric example with Ti at the centre, six hydrogens deriving from a terminal borane as the hexacoordinate planar motif and two axial P ligands (PEDJOY[8], DOI: 10.5517/ccb0d6x).
I hope this short journey through hexacoordinate planar transition metal complexes has revealed at least a flavour of the diversity in this category. I am also going to take some gentle issue with the C&E News reporting of this molecule, “Scientists proposed a hexagonal planar geometry more than 100 years ago, but it has never been captured in crystal form until now” (referring to the 2019 article which inspired this blog[1]). As I hope I have shown, a number of the examples above in crystal form actually emerged rather earlier than 2019!
References
- M. Garçon, C. Bakewell, G.A. Sackman, A.J.P. White, R.I. Cooper, A.J. Edwards, and M.R. Crimmin, "A hexagonal planar transition-metal complex", Nature, vol. 574, pp. 390-393, 2019. http://dx.doi.org/10.1038/s41586-019-1616-2
- V.G. Albano, M.C. Iapalucci, G. Longoni, L. Manzi, and M. Monari, "Synthesis of [Au3Fe2(CO)8(dppm)]- and [Au5Fe2(CO)8(dppm)2]+: X-ray Structures of [NEt4][Au3Fe2(CO)8(dppm)] and [Au5Fe2(CO)8(dppm)2][BF4]", Organometallics, vol. 16, pp. 497-499, 1997. http://dx.doi.org/10.1021/om960850g
- R. Ahlrichs, D. Fenske, H. Oesen, and U. Schneider, "Synthesis and Structure of [Ni(PtBu6)] and [Ni5(PtBu)6(CO)5] and Calculations on the Electronic Structure of [Ni(PtBu)6] and (PR)6, R = tBu,Me", Angewandte Chemie International Edition in English, vol. 31, pp. 323-326, 1992. http://dx.doi.org/10.1002/anie.199203231
- E. Hey‐Hawkins, M. Pink, H. Oesen, and D. Fenske, "Synthesen und Charakterisierung von [Ni(tBuAs)6] und [Pd(tBuAs)6]", Zeitschrift für anorganische und allgemeine Chemie, vol. 622, pp. 689-691, 1996. http://dx.doi.org/10.1002/zaac.19966220420
- S.C.K. Hau, M.C. Yeung, V.W. Yam, and T.C.W. Mak, "Assembly of Heterometallic Silver(I)–Copper(I) Alkyl-1,3-diynyl Clusters via Inner-Core Expansion", Journal of the American Chemical Society, vol. 138, pp. 13732-13739, 2016. http://dx.doi.org/10.1021/jacs.6b08674
- M. Tanabe, R. Yumoto, T. Yamada, T. Fukuta, T. Hoshino, K. Osakada, and T. Tanase, "Planar PtPd3 Complexes Stabilized by Three Bridging Silylene Ligands", Chemistry – A European Journal, vol. 23, pp. 1386-1392, 2016. http://dx.doi.org/10.1002/chem.201604502
- J. Kim, and K. Kim, "NEW THREE DIMENSIONAL [Cd(CN)2]n FRAMEWORK FORMED WITH CADMIUM CYANIDE AND Cd(CN)2·(18-CROWN-6): CRYSTAL STRUCTURE OF [Cd(CN)2]·1/2[Cd(CN)2 (18-CROWN-6)]·3/2EtOH+", Journal of Coordination Chemistry, vol. 37, pp. 7-15, 1996. http://dx.doi.org/10.1080/00958979608023536
- D.M. Goedde, and G.S. Girolami, "Titanium(II) and Titanium(III) Tetrahydroborates. Crystal Structures of [Li(Et2O)2][Ti2(BH4)5(PMe2Ph)4], Ti(BH4)3(PMe2Ph)2, and Ti(BH4)3(PEt3)2", Inorganic Chemistry, vol. 45, pp. 1380-1388, 2006. http://dx.doi.org/10.1021/ic051556w