I noted in an earlier blog, a potential (if difficult) experimental test of the properties of the singlet state of dicarbon, C2. Now, just a few days ago, a ChemRxiv article has been published suggesting another (probably much more realistic) test.[1] This looks at the so-called 7Σ open shell state of the molecule where three electrons from one σ and two π orbitals are excited into the corresponding σ* and π* unoccupied orbitals. The argument is presented that these states are not dissociative, showing a deep minimum and hence a latent quadruple bonding nature. They also note that the isoelectronic BN molecule IS dissociative.† Thus to quote: “Hence, the proof of existence of a minimum in the 7Σu+ for C2 and the absence of such a minimum in the equivalent case for BN is likely to corroborate our findings on quadruple bonding in these two cases.“
Although a PES (potential energy surface) is shown for 7Σ C2, no vibrational wavenumber is reported. So in the spirit of a commentary on this pre-print, I have calculated these values (CCSD(T)/Def2-TZVPP) for the molecules noted in the article and a few other isoelectronic species. The results are collected at DOI: 10.14469/hpc/6599
| Property | BeC2- | BB2- | BC1- | BN | CC | CN1+ | NN2+ | BeO | BO1+ | LiF |
|---|---|---|---|---|---|---|---|---|---|---|
| Bond length, Å |
2.281 | 1.858 | 1.768 | 3.216 | 1.585 | 1.638 | 1.523 | 2.602 | 200.7 | 2.179 |
| Bond stretch, cm-1 |
182 | 638 | 699 | 48 | 1055 | 759 | 934 | 363 | 0.4 | 626‡ |
The two singly occupied σ-orbitals are shown below and are in part responsible for the non-dissociative behaviour. 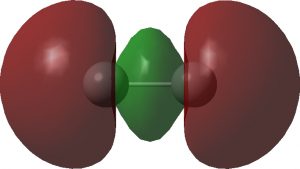
The results using this method (the article reports many more methods), reveal that C2 does indeed exist in a minimum for the heptet state, whilst the isoelectronic BN is only very weakly bound, in effect dissociative. Another dissociative isoelectronic is BO1+, whilst N22+ despite the coulombic repulsions of a double positive charge, still manages to be bound with a relatively short bond length. A further neutral diatomic BeO is also clearly non-dissociative.
Lets hope a spectroscopist somewhere tries to take a look at these heptet excited states of such molecules so that further experimental insight can be cast on this fascinating problem.
‡The orbital occupancy of this species is different from the others. †As are the neutral ten valence electron N≡N (confirmed here) and HC≡CH.
References
- I. Bhattacharjee, D. Ghosh, and A. Paul, "Resolving the Quadruple Bonding Conundrum in C2 Using Insights Derived from Excited State Potential Energy Surfaces: A Molecular Orbital Perspective", 2019. http://dx.doi.org/10.26434/chemrxiv.11446224.v1
Tags: Interesting chemistry