Increasingly, individual small molecules are having their structures imaged using STM, including cyclo[18]carbon that I recently discussed. The latest one receiving such treatment is Kekulene.[1]
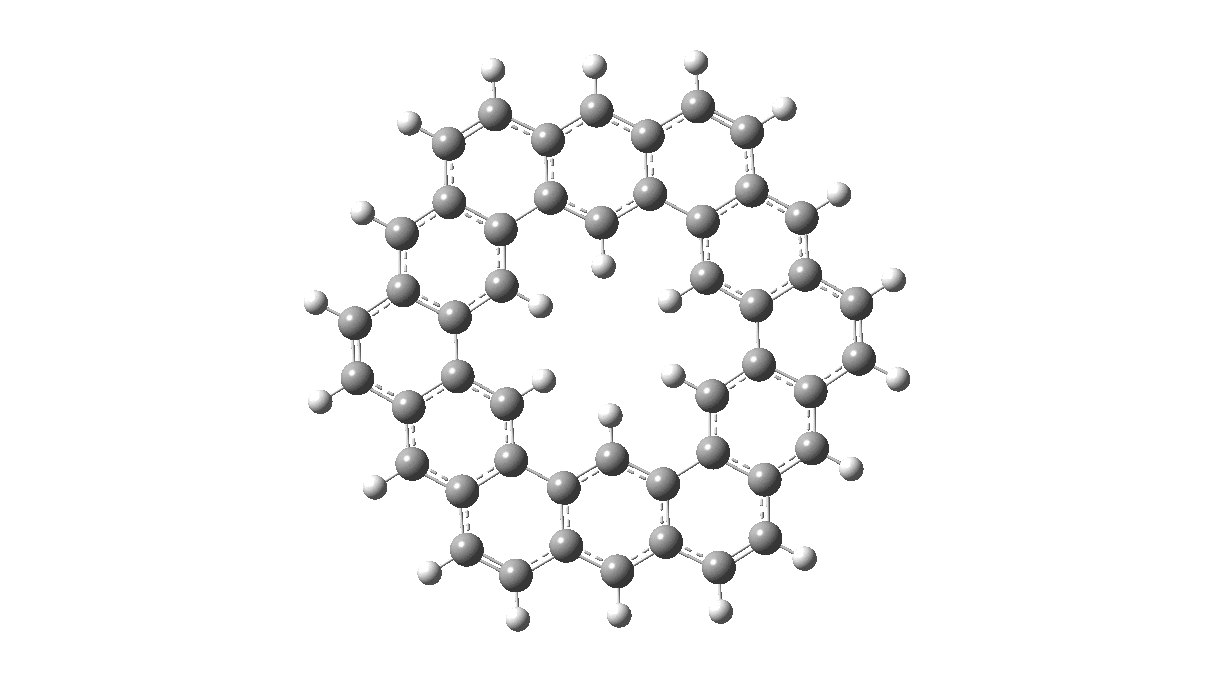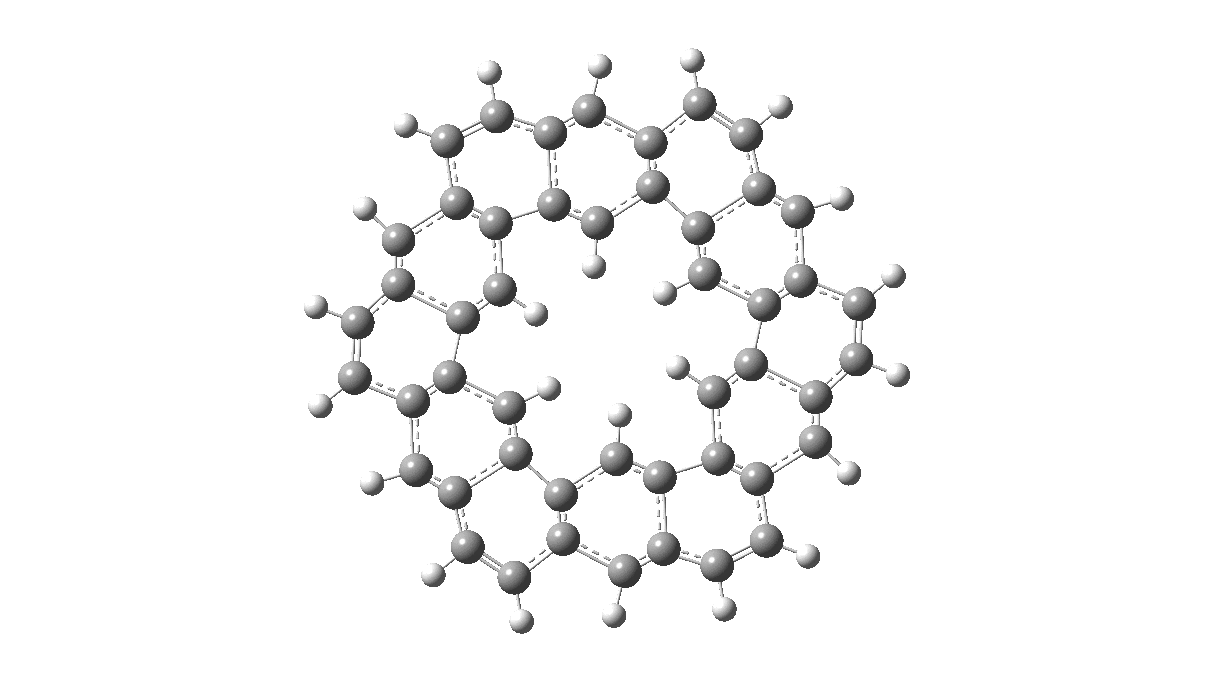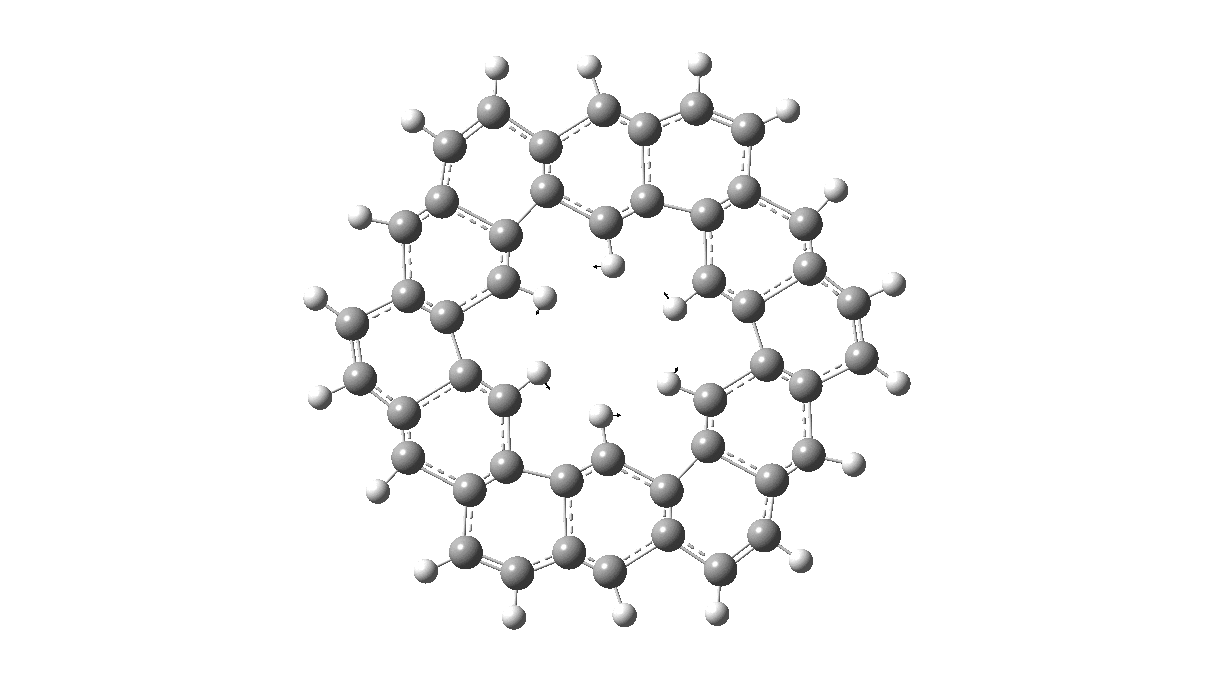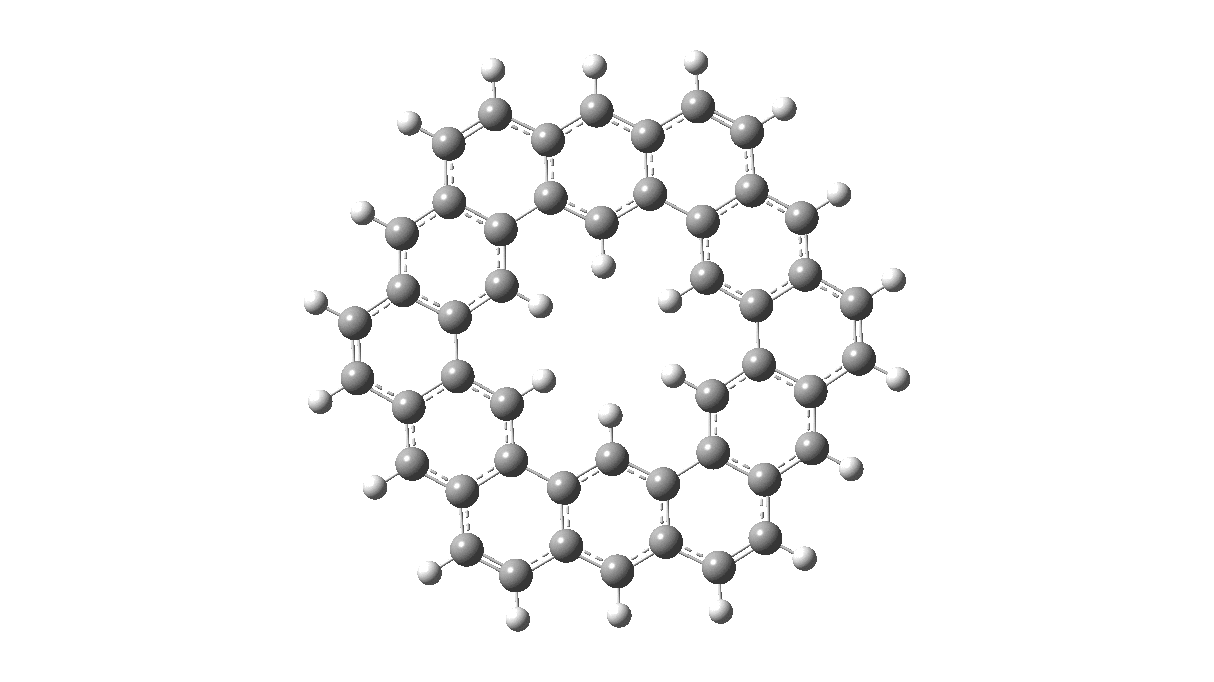
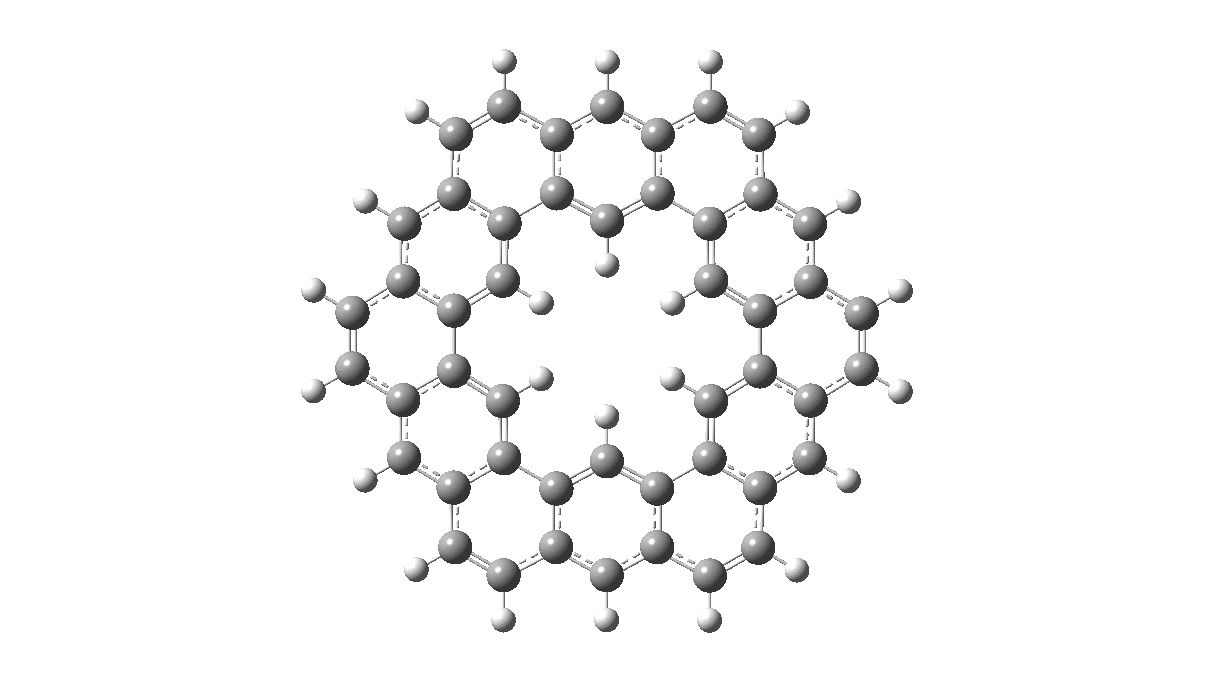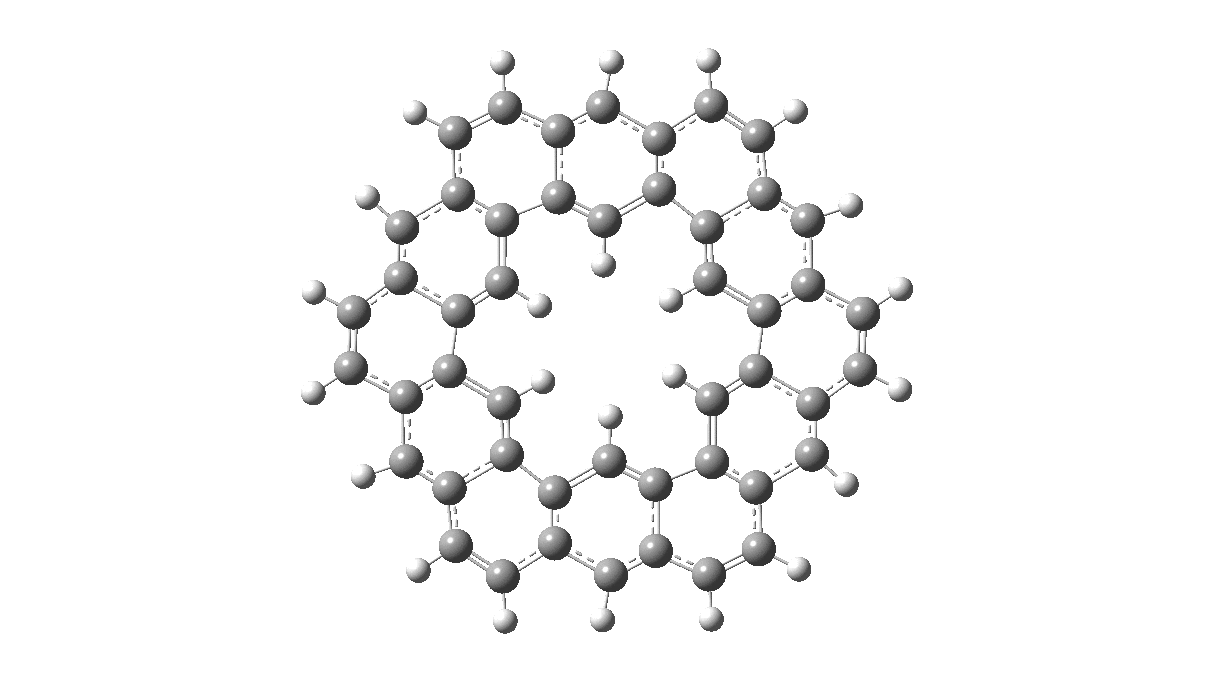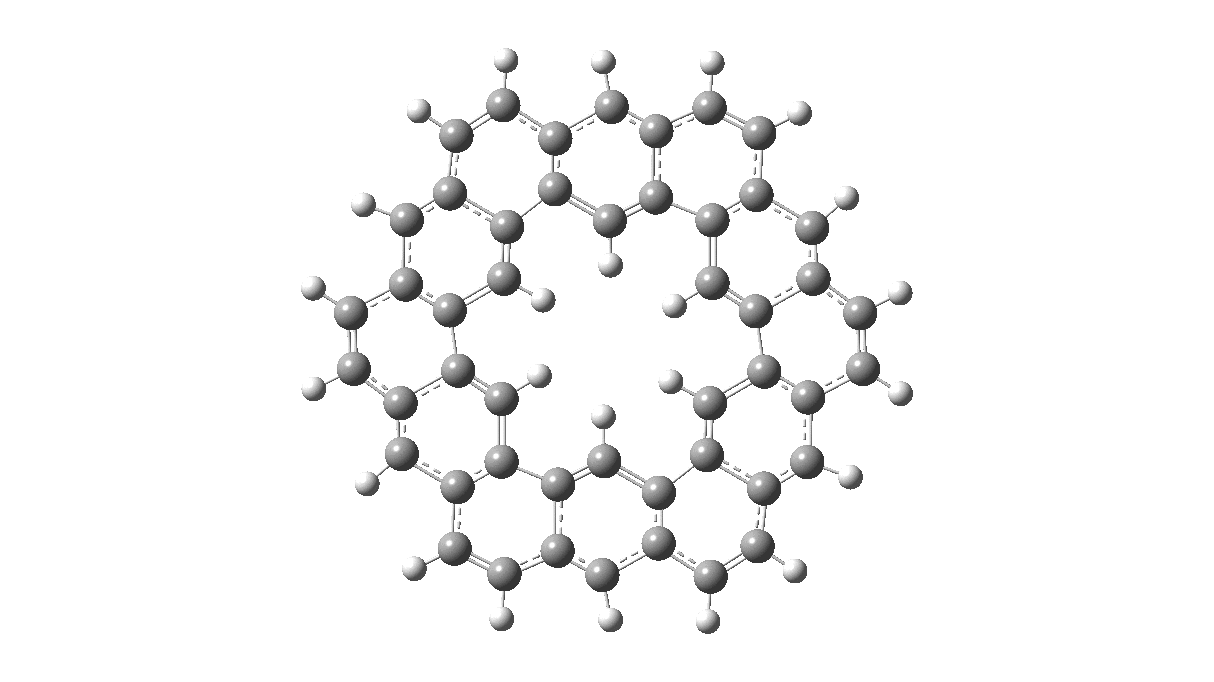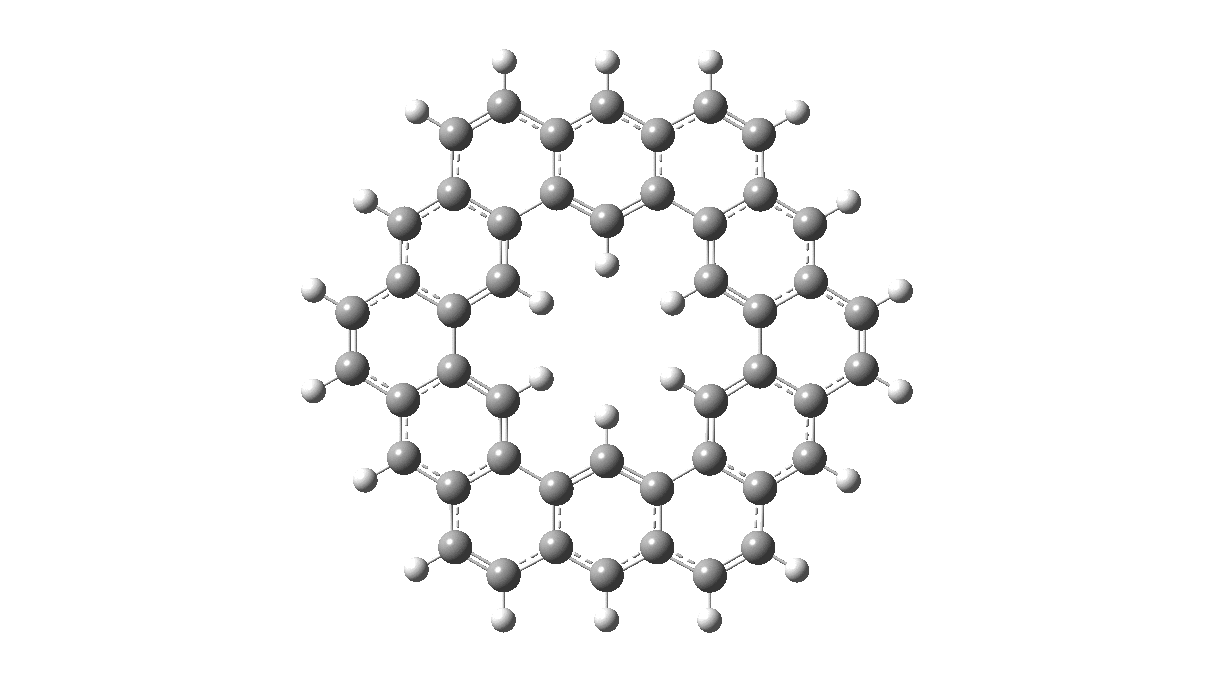
As with cyclo[18]carbon, the point of interest was which of the two resonance structures shown below most closely resembled the measured structure. The one on the left has six cyclohexatriene motifs (red), also known as Clar rings (although I have argued they could also be called Armstrong rings), with six largely isolated double bonds. These each follow the 4n+2 π-electron aromaticity rule (n=1). The structure on the right has instead two fully conjugated larger rings, the inner one blue following 4n+2, n=4 (18) and the outer one (magenta) following 4n+2, n=7 (30).
Now, the STM result[1] clearly came down in favour of the left hand structure, with a sextet of six-rings, rather than the larger annulenes implied by the right hand structure. A previously reported crystal structure[2] came to a similar conclusion, and the species is shown as fully planar. There is just one oddity about this structure. The distance between the inner six hydrogens is reported as 1.938Å. In reality, it must be shorter, since the C-H bond lengths of these hydrogens are reported as 1.00Å whereas a more realistic value is ~1.09Å. Applying this well-known correction means that the non-bonded H-H distance in Kekulene is actually closer to 1.83Å, which is unusually close. Recollect a similar issue arose in the imaging of purportedly planar tetraphenylporphrin, in which H…H distances as short as 0.8Å were implied in a published article.[3]
Previously, I have shown that conjugated π-electron rings can exhibit Kekulé vibrational modes for all these ring sizes, and so the question arises as to the nature of any Kekulé vibration in Kekulene itself. This vibration corresponds to a motion which creates two alternative cyclohexatriene structures (for benzene itself) from the symmetrical molecule. For benzene, this mode is real, ie it takes energy to distort benzene into a cyclohexatriene. For larger annulenes, this vibration is imaginary and hence corresponds to a transition state between two bond alternating lower energy forms. So the Kekulé vibration in Kekulene should be distinctly different for the two structures shown above. I am using two different density functional methods, B3LYP+GD3BJ/Def2-TZVPP and ωB97XD/Def2-TZVPP (FAIR DOI: 10.14469/hpc/6226), which probably straddle reality in terms of their propensity for bond length alternation (BLA) in larger annulene rings.
The results show that a fully planar molecule with D6h symmetry is actually a transition state in which three of these six inner hydrogen atoms move up and three down, giving a non-planar molecule with D3d symmetry and increasing the H…H separation. However, both DFT methods also agree that there is just one Kekulé vibration, which is real (ν(A2g) 1389/B3LYP or 1395/ωB97XD cm-1) and corresponds to the below.
All six 6-membered Clar/Armstrong rings are executing their own Kekulé vibration, but in perfect synchrony with each other. There is almost no activity in the six passive double bonds. There is certainly no extended Kekulé vibration similar to the one shown here which involves the entire larger ring, whether in the inner or outer periphery.
So these results match nicely with the initial crystal structure and the recent STM imaging one Curiously, no-one had ever mentioned the Kekulé vibration in this species, and so this omission is rectified here.
Update: Some variations on the theme above.
- The inner ring of hydrogens is replaced by carbons
This has the following Kekule modes, a B2u mode centered on just the inner six carbons (red, ν 1423 cm-1)
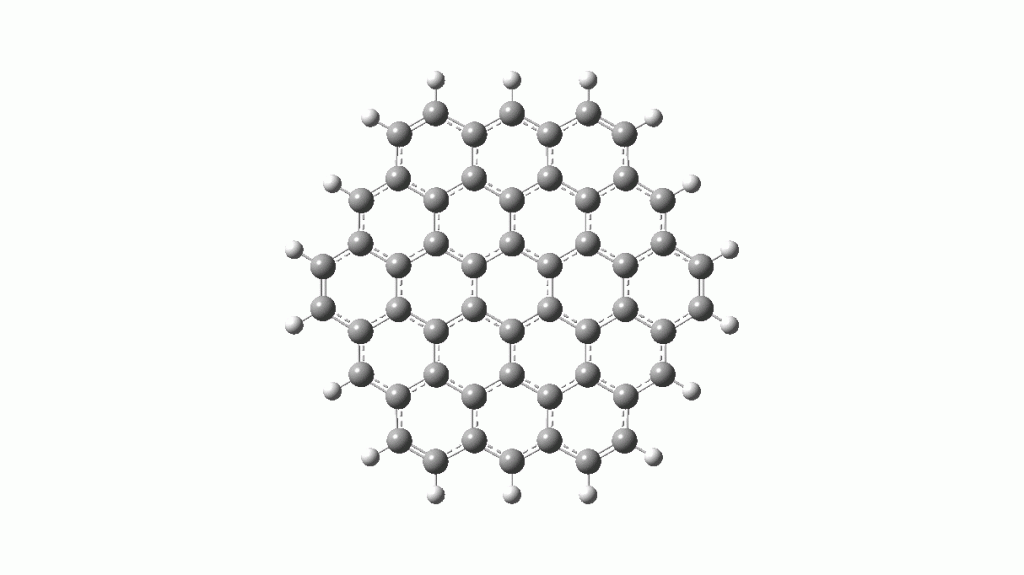
and a degenerate E2g mode (blue, ν 1383 cm-1) for the six outer Clar rings.
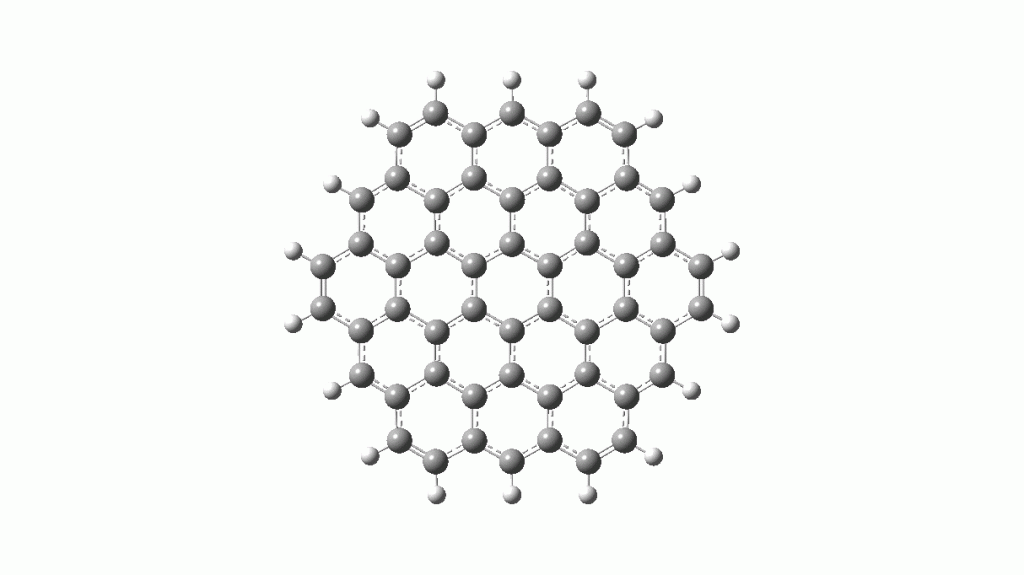
- The inner ring is replaced by borons.
References
- I. Pozo, Z. Majzik, N. Pavliček, M. Melle-Franco, E. Guitián, D. Peña, L. Gross, and D. Pérez, "Revisiting Kekulene: Synthesis and Single-Molecule Imaging", Journal of the American Chemical Society, vol. 141, pp. 15488-15493, 2019. http://dx.doi.org/10.1021/jacs.9b07926
- H.A. Staab, F. Diederich, C. Krieger, and D. Schweitzer, "Cycloarenes, a New Class of Aromatic Compounds, II. Molecular Structure and Spectroscopic Properties of Kekulene", Chemische Berichte, vol. 116, pp. 3504-3512, 1983. http://dx.doi.org/10.1002/cber.19831161022
- J. Lee, K.T. Crampton, N. Tallarida, and V.A. Apkarian, "Visualizing vibrational normal modes of a single molecule with atomically confined light", Nature, vol. 568, pp. 78-82, 2019. http://dx.doi.org/10.1038/s41586-019-1059-9
Tags: Interesting chemistry
I saw your posts at In The Pipeline. Are you able to comment on whether kekulene can be reduced to a stable (kekulene)(6-) or oxidized to a stable (kekulene)(2+)?
Thank you.