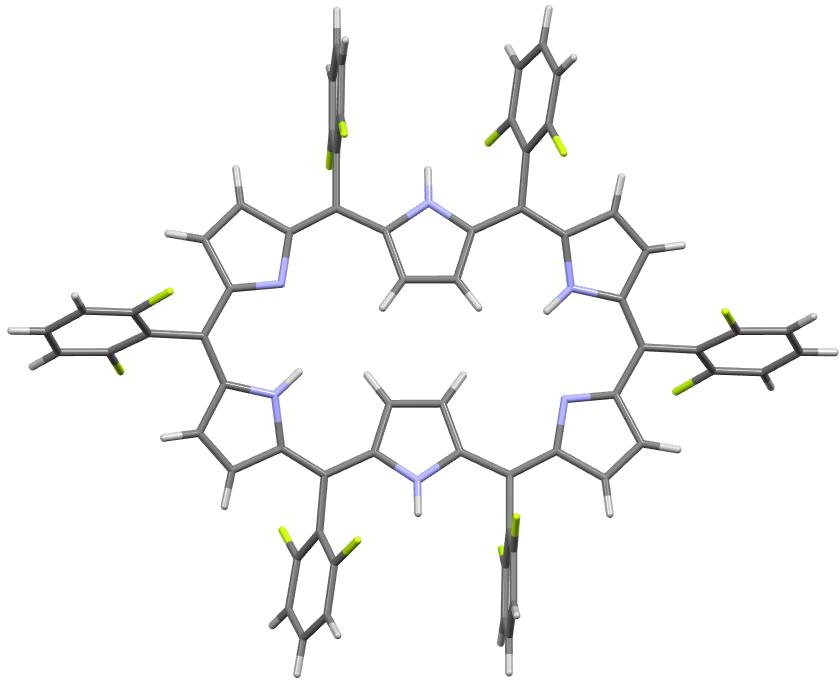
In the previous post, I looked at a class of molecule known as hexaphyrins, inspecting bond length alternation (BLA) at the so-called meso position, the carbon atom joining two pyrrole rings. A search of the difference in bond lengths at this position had shown two significant clusters of crystal structures.
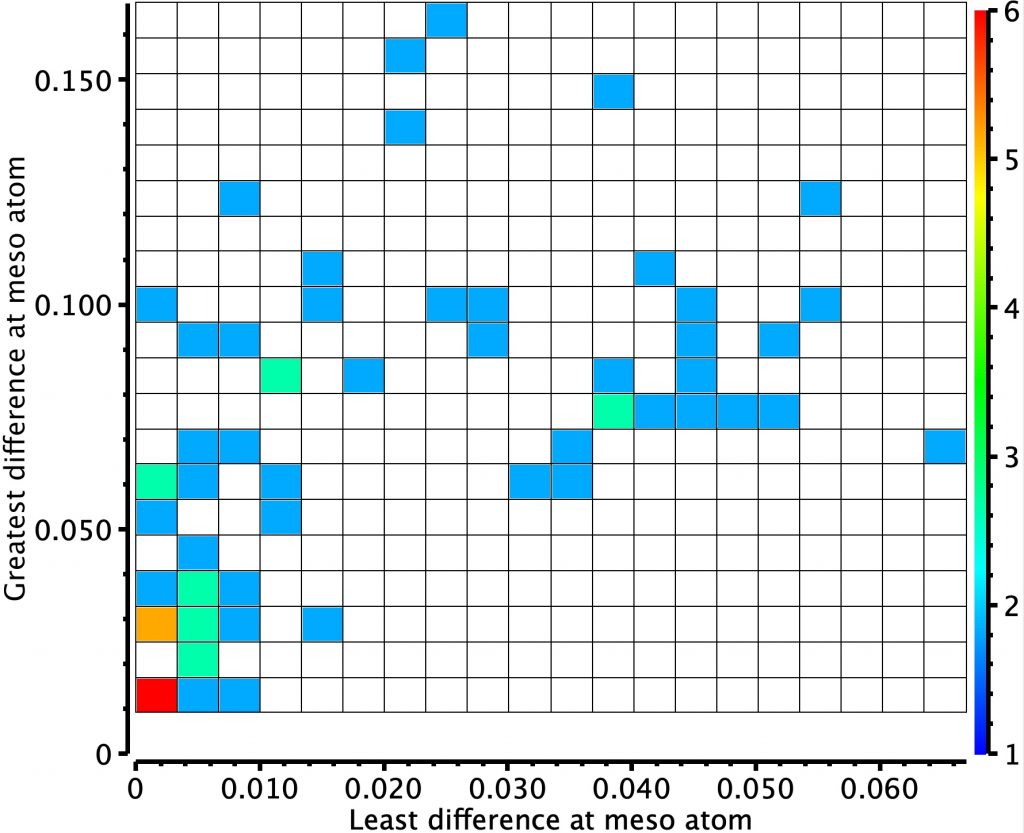 Molecules in the bottom left of this diagram shows little or no bond length alternation. The right middle shows another cluster with more extreme (and unequal) bond length alternation. I have selected one molecule from this cluster, EGIJEK and it differs from EGIHUY in having four NH units in the ring, whereas the latter has only two.
Molecules in the bottom left of this diagram shows little or no bond length alternation. The right middle shows another cluster with more extreme (and unequal) bond length alternation. I have selected one molecule from this cluster, EGIJEK and it differs from EGIHUY in having four NH units in the ring, whereas the latter has only two.
The effect this has is illustrated below. A pyrrole with an NH group contributes two π-electrons to the conjugated periphery whereas a pyrrole with just an N contributes only one. Thus the four NH rings in EGIJEK contribute overall two more π-electrons to the periphery than EGIHUY with just two NH rings. This increases the π-electron count from 26 to 28, driving EGIJEK from a 4n+2 into a 4n π-electron count (n=7) and making it formally anti-aromatic. This in turn induces strong bond length alternation (as for example in cyclobutadiene).
Here is a reprise of the bond length table shown in the previous post, but for EGIJEK.
| Meso distances, Å | abs(Δr) | |
|---|---|---|
| EGIJEK crystal, Ci symmetry, DOI: 10.5517/ccrts2d | ||
| 1,43657 | 1.37034 | 0.06623 |
| 1.35661 | 1.44895 | 0.09234 |
| 1.42356 | 1.37287 | 0.05069 |
| B3LYP+GD3BJ/Def2-SVPP (FAIR DOI: 10.14469/hpc/6194) | ||
| 1.44019 | 1.38259 | 0.0576 |
| 1.37732 | 1.44481 | 0.06749 |
| 1.43261 | 1.38568 | 0.04693 |
| ωB97XD/Def2-SVPP (FAIR DOI: 10.14469/hpc/6194 ) | ||
| 1.44575 | 1.37537 | 0.07038 |
| 1.36214 | 1.45508 | 0.09294 |
| 1.44221 | 1.36958 | 0.07263 |
Of the two functionals used in the calculations, the ωB97XD form slightly over-estimates the BLA, with the B3LYP slightly under-estimating it. This seems to tally with the earlier observations made for cyclo[n]carbons.
Perhaps these sorts of molecules might form useful reality checks on calculating bond length alternations in large ring cyclic conjugated molecules.