The theme of the last three posts derives from the recently reported claimed experimental observation of bond length alternation (BLA) in cyclo[18]carbon, a ring of just 18 carbon atoms.[1] Having found that different forms of quantum calculation seem to find this property particularly difficult to agree upon, not only for cyclocarbon but for twisted lemniscular annulenes (which contain CH rather than just C units), I thought it might be time to look at some more experimental data and my chosen system is a class called the hexaphyrins, of which there are a number of experimental crystal structures.
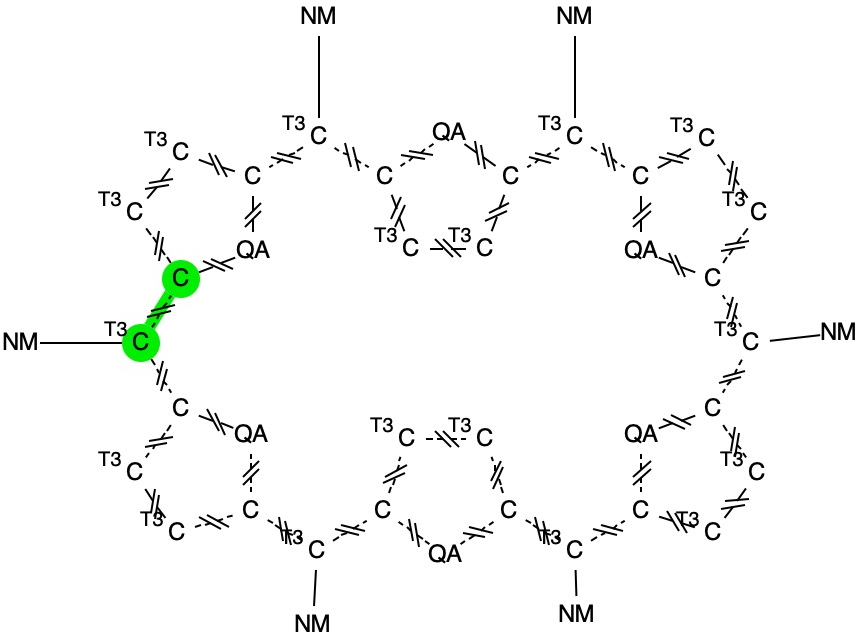
The general form these molecules take is shown below. Here, QA can be N or S and the hashed bonds are defined as any type. T3 indicates an atom designated as having just three substituents. For each of the six meso carbons to which a non-metal (NM) is attached, a pair of C-C distances is specified as the search variable. These will define any bond length alternation.
A search of the current crystal structure database specifies no errors and no disorder (see FAIR DOI: 10.1021/ja801983d). For any temperature, 64 hits are obtained. Specifying a temperature of < 95K and an R factor of <7.5% reduces the number to 18. The six pairs of distances are then processed for each molecule as follows;
- The Abs() operator is chosen and the absolute value of the difference in values between each of the six pairs is calculated, using the minus operator.
- Then using the Least() and Greatest() operators, the corresponding values for each of the six differences is calculated.
- Finally, a Heat plot of the least vs the greatest values is constructed for each of the molecules.
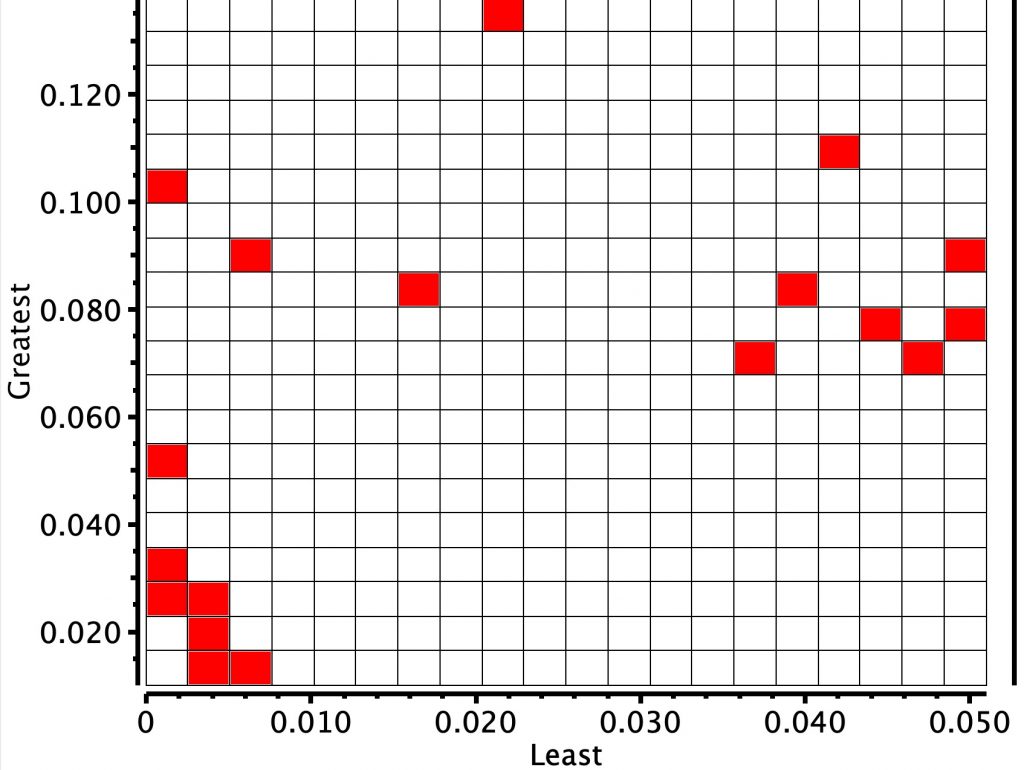
 The result is shown below for the first search
The result is shown below for the first search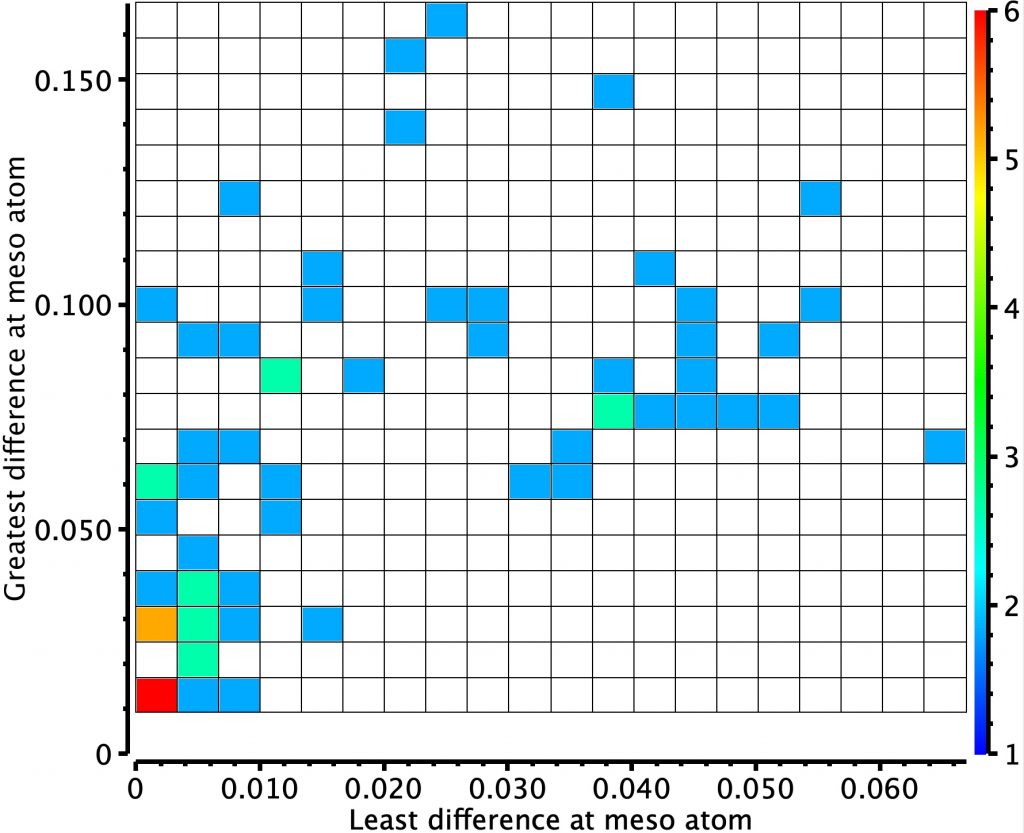
There are two main clusters.
- The cluster with the hotspot (red) shows that the minimum and maximum BLA (bond length alternations) at any of the six meso carbon atoms is < 0.01Å.
- There is a second more diffuse cluster for which a greater minimum BLA at any meso atom of ~0.045Å and the maximum ~0.10Å are recorded.
- The most diffuse cluster is where the minimum distance is 0.01Å but the maximum is again ~0.10Å.
This result suggests that BLA may be sensitive to the nature of the substituents on the ring. Cluster 1 above, with the least BLA, may also arise because the crystal structure is actually the average of two BLA forms. We can reduce the temperature of the measurements to e.g. < 95K to see the effect this might have on any dynamically averaging effect on the distances. A very similar distribution can be seen (below).
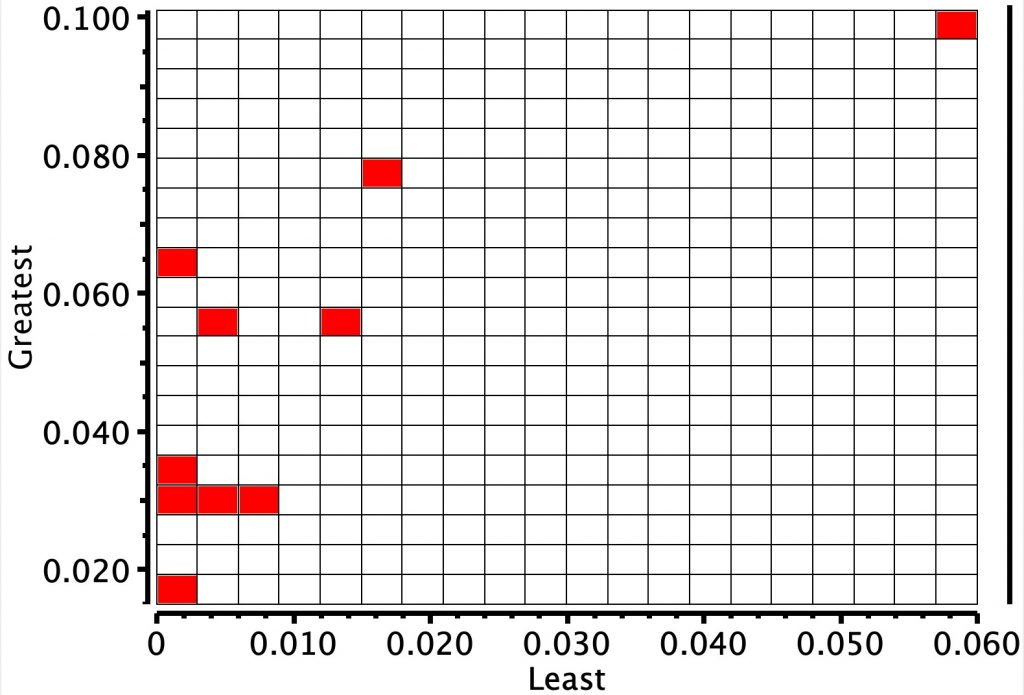
Next, an equivalent search for octaphyrins. Although there are fewer examples, the same clustering is seen.
The compound (code EGIHUY, a [26]phyrin, where 26 = 4n+2 = aromatic, DOI: 10.5517/ccrts0b[2]) in the red hot spot in the top diagram, with the smallest BLA was chosen for computation (FAIR data DOI: 10.14469/hpc/6179). This has Ci symmetry and so only three pairs of distances need to be considered.
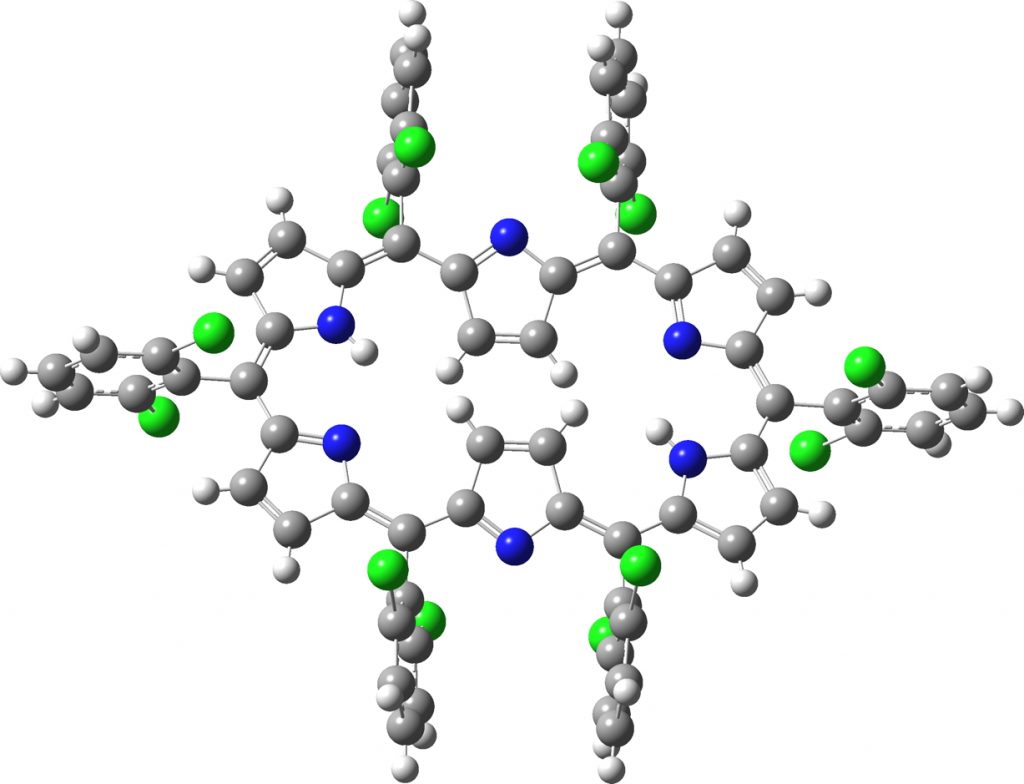
Click image for 3D model
The results show that both B3LYP and ωB97XD DFT methods actually get pretty good geometries, reproducing the non-bond-alternating characteristics of this hexaphyrin. This in turn suggests that the absence of BLA may be real rather than a crystallographic averaging artefact.
| Meso distances, Å | abs(Δr) | |
|---|---|---|
| EGIHUY crystal, Ci symmetry | ||
| 1.39930 | 1.40168 | 0.00248 |
| 1.41029 | 1.39960 | 0.01069 |
| 1.40874 | 1.39902 | 0.00972 |
| B3LYP+GD3BJ/Def2-TZVPP | ||
| 1.40842 | 1.40058 | 0.00784 |
| 1.39972 | 1.40963 | 0.00991 |
| 1.40848 | 1.40614 | 0.00234 |
| ωB97XD/Def2-TZVPP | ||
| 1.39636 | 1.40513 | 0.00877 |
| 1.40117 | 1.40680 | 0.00563 |
| 1.40730 | 1.39510 | 0.0122 |
What has been achieved? Well, the crystal structures show that whilst some hexaphyrin molecules have no bond alternation, most in fact do. DFT calculations reproduce the lack of bond alternation in one molecule. The next step is to show whether they also reproduce those which do have BLA. The story is not ended yet!
References
- K. Kaiser, L.M. Scriven, F. Schulz, P. Gawel, L. Gross, and H.L. Anderson, "An sp-hybridized molecular carbon allotrope, cyclo[18]carbon", Science, vol. 365, pp. 1299-1301, 2019. http://dx.doi.org/10.1126/science.aay1914
- J. Sankar, S. Mori, S. Saito, H. Rath, M. Suzuki, Y. Inokuma, H. Shinokubo, K. Suk Kim, Z.S. Yoon, J. Shin, J.M. Lim, Y. Matsuzaki, O. Matsushita, A. Muranaka, N. Kobayashi, D. Kim, and A. Osuka, "Unambiguous Identification of Möbius Aromaticity formeso-Aryl-Substituted [28]Hexaphyrins(1.1.1.1.1.1)", Journal of the American Chemical Society, vol. 130, pp. 13568-13579, 2008. http://dx.doi.org/10.1021/ja801983d
Tags: Interesting chemistry