Stereo-induction is, lets face it, a subtle phenomenon. The ratio of two stereoisomers formed in a reaction can be detected very accurately by experiment, and when converted to a free energy difference using ΔG = -RT Ln K, this can amount to quite a small value (between 0.5 – 1.5 kcal/mol). Can modelling reproduce effects originating from such small energy differences? Well one system that has been argued about now for several decades is shown below as 1.
Way back in 1992, we thought that the explanation for attack by an electrophile on the alkene from the syn face was electrostatic (although it did depend on the nature of the electropile; thus we concluded that attack by Hg(OH)2 was electrostatic, but by BH3 was orbital controlled). Recently, a different explanation has emerged, relating to how the fundamental normal vibrational modes of the molecule interact with the transition normal mode for the reaction. A new example of this, relating to reaction of the isomeric 2 with a peracid has recently been discussed on Steve Bachrach’s blog. Here, the peroxide of the peracid is thought to act as an electrophile (although one must bear in mind that it does bear two electron lone pairs!). The conclusion was pretty clear cut; the experimental preference for syn (92%) over the anti isomer (8%, ΔΔG = 1.4 kcal/mol) was NOT due to electrostatic effects, but due to distorsional asymmetry in the vibrational mode that couples/forms the transition state mode.
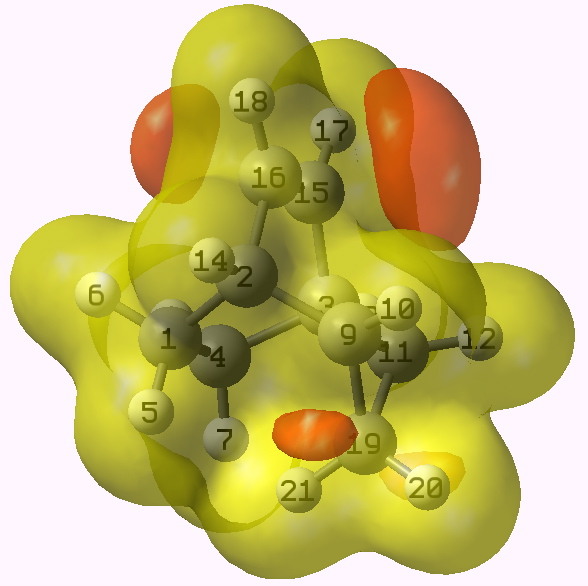
I could not resist revisiting this system. As in 1992, a molecular electrostatic potential was calculated for 2. The method used was wB97XD/aug-cc-pvdz, and if you want the archive of this calculation to evaluate it yourself, see here).

MEP for 2. Click on diagram for 3D.

The norbornene with a four-membered ring
I would conclude by saying that it can be ferociously difficult to identify the fundamental origins of stereo-induction. But I leave the argument in the hands of the reader now. What do you think?
Tags: chiroptical, energy differences, free energy difference, hybridization, interaction energy, Interesting chemistry, Steve Bachrach

Interesting original paper and interesting analysis here. What is attractive to organic chemists about analysing sterics and some simple electronic arguments when explaining stereoselectivity is that such explanations are “intuitive” and simple, without needing a computer, permitting back-of-the-envelope predictions. The paper authors say “The model is understood in terms of readily identifiable molecular structure parameters and allows qualitative analysis without the need to resort to computational methods” but I’m not so sure. The analysis (paper and yours) is complex – there’s no getting away from that – but is none the worse for it. One needs an ipad rather than a pencil. The authors do provide this nice summary, however: “In this way, stereoinduction by distortional asymmetry is analogous to face selectivity in instances where anchimeric assistance is operative (see the stereoselectively for the 7-norbornenyl cation). Hence the reagent will approach the π face from the side opposite the strongest orbital interactions.”