There is a predilection amongst chemists for collecting records; one common theme is the length of particular bonds, either the shortest or the longest. A particularly baffling type of bond is that between the very electronegative F atom and an acid hydrogen atom such as that in OH. Thus short C-N…HO hydrogen bonds are extremely common, as are C-O…HO.‡ But F atoms in C-F bonds are largely thought to be inert to hydrogen bonding, as indicated by the use of fluorine in many pharmaceuticals as inert isosteres.[1] Here I do an up-to-date search of the CSD crystal structure database, which is now on the verge of accumulating 1 million entries, to see if any strong C-F…HO hydrogen bonding may have been recently discovered.
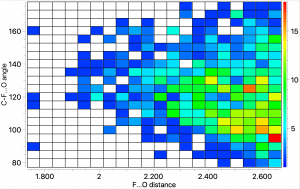
The search query uses the CF…HO distance as one variable, and the C-F-H angle as the second. The first diagram shows just intermolecular interactions, up to a distance of 2.7Å which is the sum of the van der Waals radii of the two elements. The hot spot occurs at this value, and an angle of ~95°.
 The intra-molecular plot shows a similar value for the most common F…H distance, with the interesting variation that the angle subtended at F is about 80°.
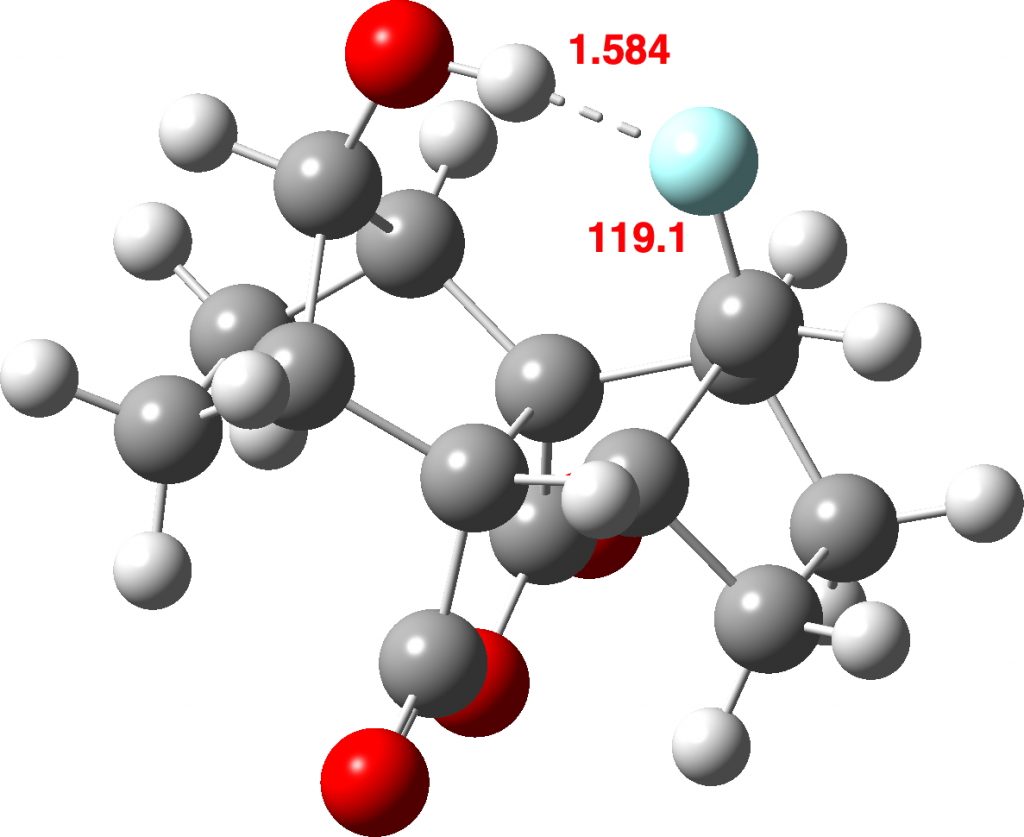
The intra-molecular plot shows a similar value for the most common F…H distance, with the interesting variation that the angle subtended at F is about 80°.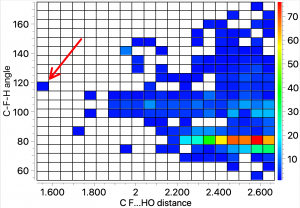 The outlier at the short end of the spectrum (arrow) was observed in 2014[2] with the structure shown below. It is indeed the current record holder by some margin! This length by the way is however a great deal longer than the shortest O…HO hydrogen bonds, which can be in the region of 1.2Å (with the proton sometimes symmetrically disposed between the two oxygen atoms). The value is also very similar to the record holder for the shortest C-H…H-C interaction.
The outlier at the short end of the spectrum (arrow) was observed in 2014[2] with the structure shown below. It is indeed the current record holder by some margin! This length by the way is however a great deal longer than the shortest O…HO hydrogen bonds, which can be in the region of 1.2Å (with the proton sometimes symmetrically disposed between the two oxygen atoms). The value is also very similar to the record holder for the shortest C-H…H-C interaction.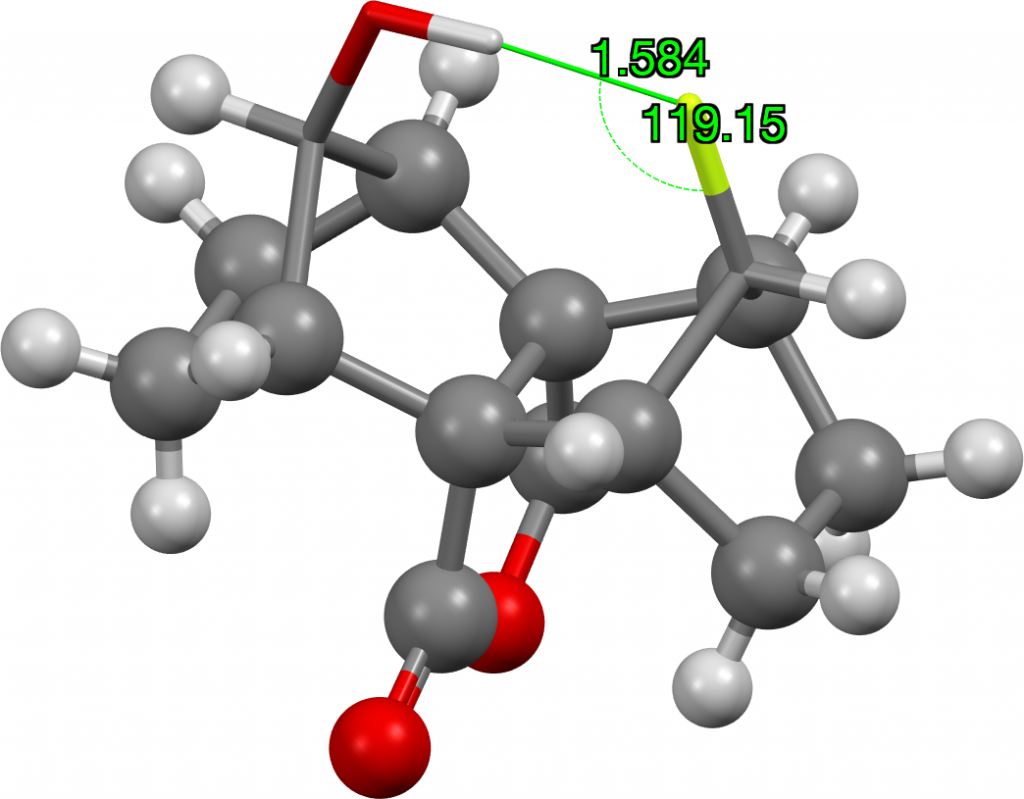
It is always useful to check up on crystallographic hydrogen atom positions using a quantum calculation, so here is one at the ωB97XD/Def2-TZVPP level (Data DOI: 10.14469/hpc/5131) which replicates the values nicely.
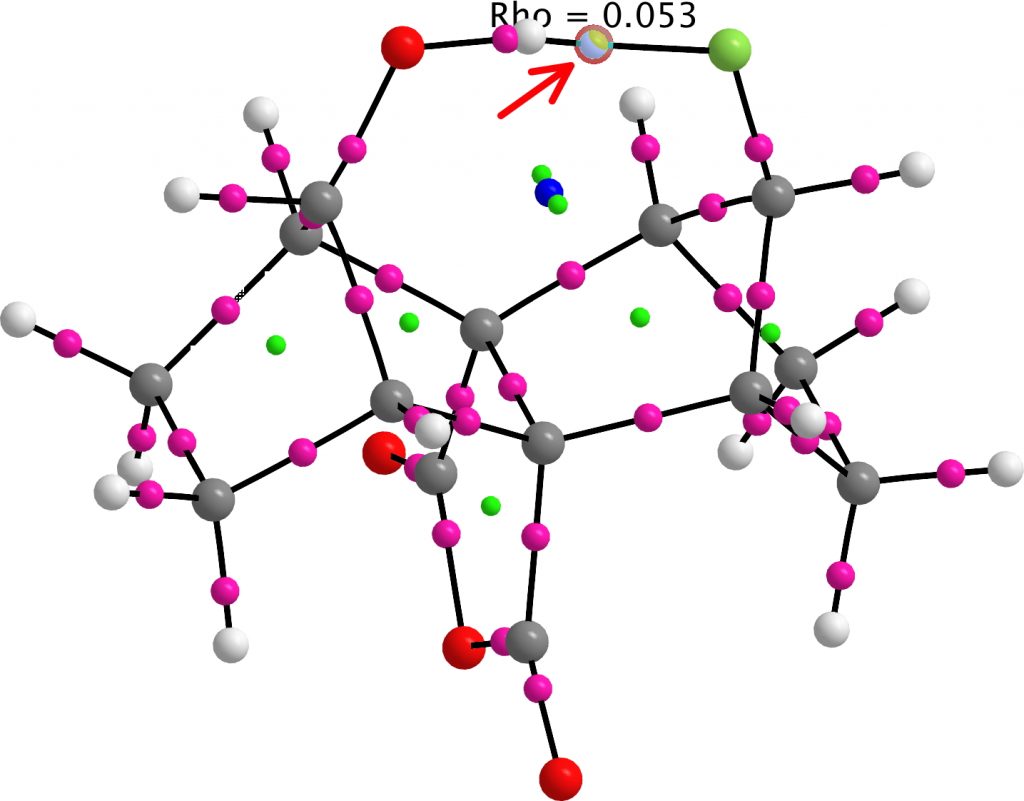
A QTAIM analysis of the critical points shows that the F…H BCP has a high value of ρ(r) (most hydrogen bonds only reach about 0.03 au).
NBO analysis indicates the E(2) perturbation energy for donation from an F lone pair into the H-O σ* orbital is 21.2 kcal/mol, which indicates a strong H-bond (typical C-O…HO values are 18-22 kcal/mol). The F…H bond order is 0.05.
This molecule has another interesting property, also noted in the original article;[2] the shift in wavenumber of the O-H stretching vibration. Most hydrogen bonds are characterised by the shift (mostly red and recently discovered blue shifts) that occurs in the OH group when it hydrogen bonds. These shifts are typically 100-200 cm-1 but in this molecule there is no shift, which is described as “exceptional”.
The 1H NMR shift of the OH proton is observed at δ 4.8 ppm, with the value calculated here (ωB97XD/Def2-TZVPP) being 4.75 ppm. A very large H-F coupling was observed of 68 Hz, again a very high value for a “through space” hydrogen bond.
So another record for the molecule makers to try to break!
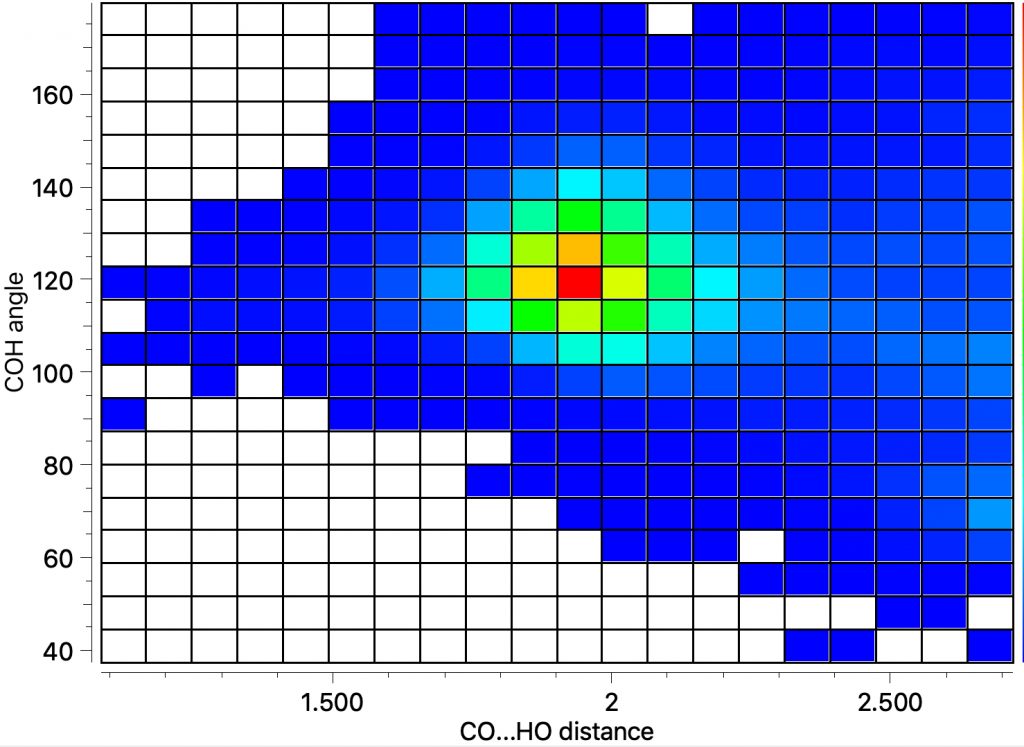
‡Respectively 7142 and 31428 intermolecular (3859 and 10602 intra) examples using the same search parameters as above, with the shortest values being ~1.28 and ~1.2Å. 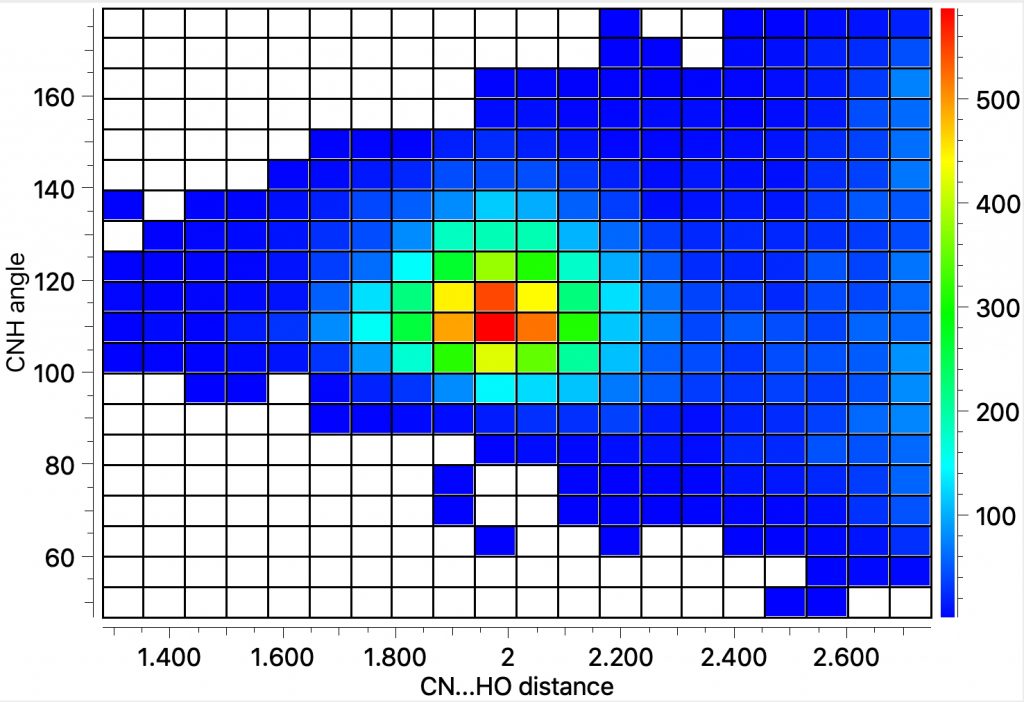
References
- S. Purser, P.R. Moore, S. Swallow, and V. Gouverneur, "Fluorine in medicinal chemistry", Chem. Soc. Rev., vol. 37, pp. 320-330, 2008. http://dx.doi.org/10.1039/B610213C
- M.D. Struble, C. Kelly, M.A. Siegler, and T. Lectka, "Search for a Strong, Virtually “No‐Shift” Hydrogen Bond: A Cage Molecule with an Exceptional OH⋅⋅⋅F Interaction", Angewandte Chemie International Edition, vol. 53, pp. 8924-8928, 2014. http://dx.doi.org/10.1002/anie.201403599
Tags: Chemical bond, chemical bonding, Chemical elements, Chemistry, Fluorine, Hydrogen, Hydrogen bond, Intermolecular forces, Natural sciences, perturbation energy, pharmaceuticals, Physical sciences, Refrigerants, search parameters, search query, Supramolecular chemistry