This is a follow-up to the post on exploring the directing influence of (electron donating) substituents on benzene[cite]10.1021/acs.jchemed.5b00346[/cite] with the focus on heteroaromatic rings such indoles, pyrroles and group 16 analogues (furans, thiophenes etc).
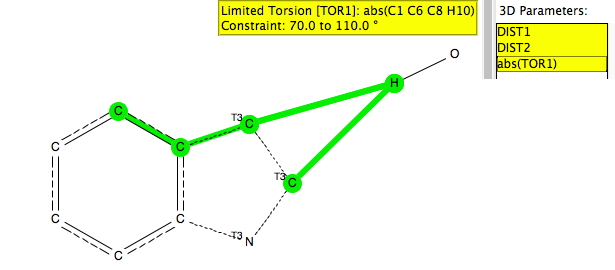
The search query is shown above (and is available here[cite]10.14469/hpc/665[/cite]). As before, the distance is compared from an electrophile, modelled as the hydrogen atom of an OH group, to both the carbon next to the heteroatom (C2) and the C3 carbon. The torsion is defined so as to ensure that the OH group is approaching the π-face of the ring. The other constraints are R < 0.1, no disorder and no errors and normalised H positions.
Firstly, indoles (as above). There are only a few hits, but even so one can see that they all cluster in the top left triangle, where the distance to C2 is always longer than to C3. Indeed, this is the known position for electrophilic substitution of indoles.
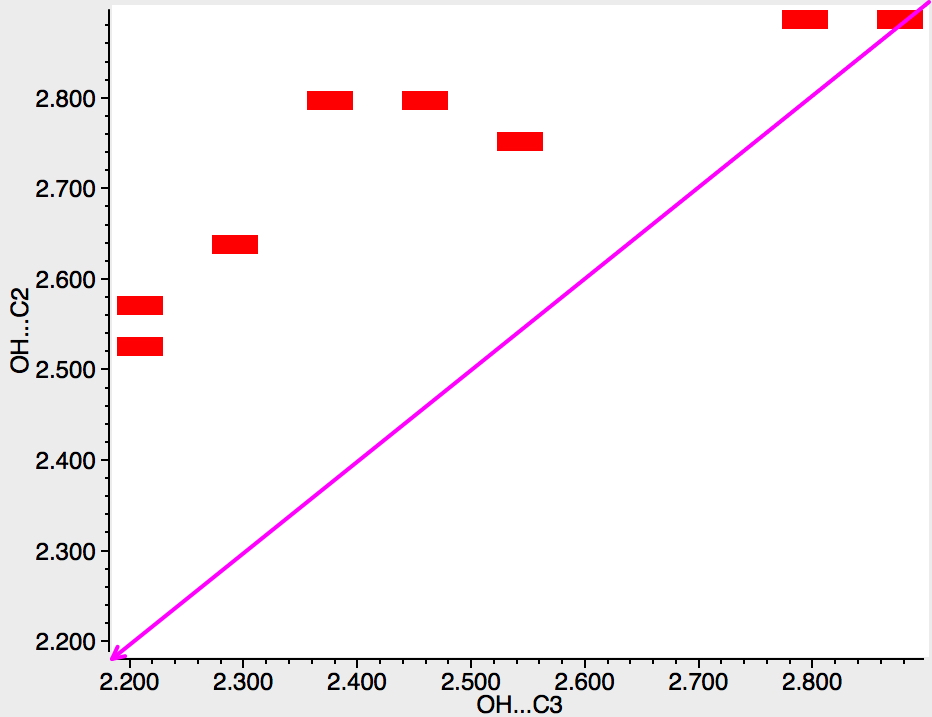
The search can be extended by removing the benzo group so as to also include pyrroles. More hits are obtained, and again most of them collect in the top left triangle. The hot spot indicates that the difference in lengths is ~0.3Å in favour of the 3-position, a very similar discrimination to that previously found for benzene groups with an electron donating substituent.
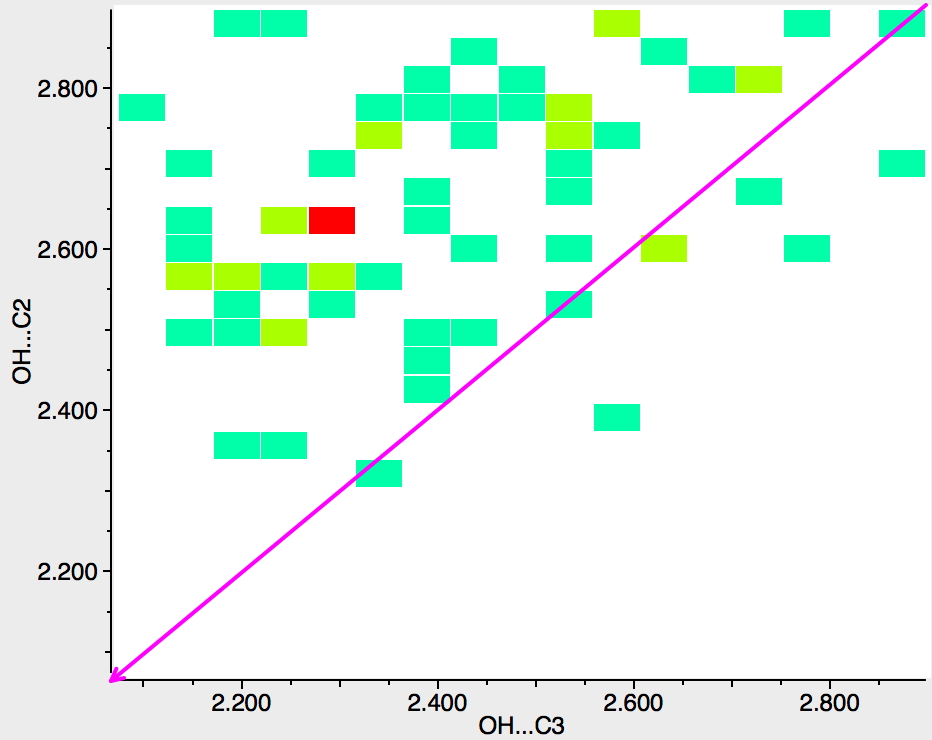
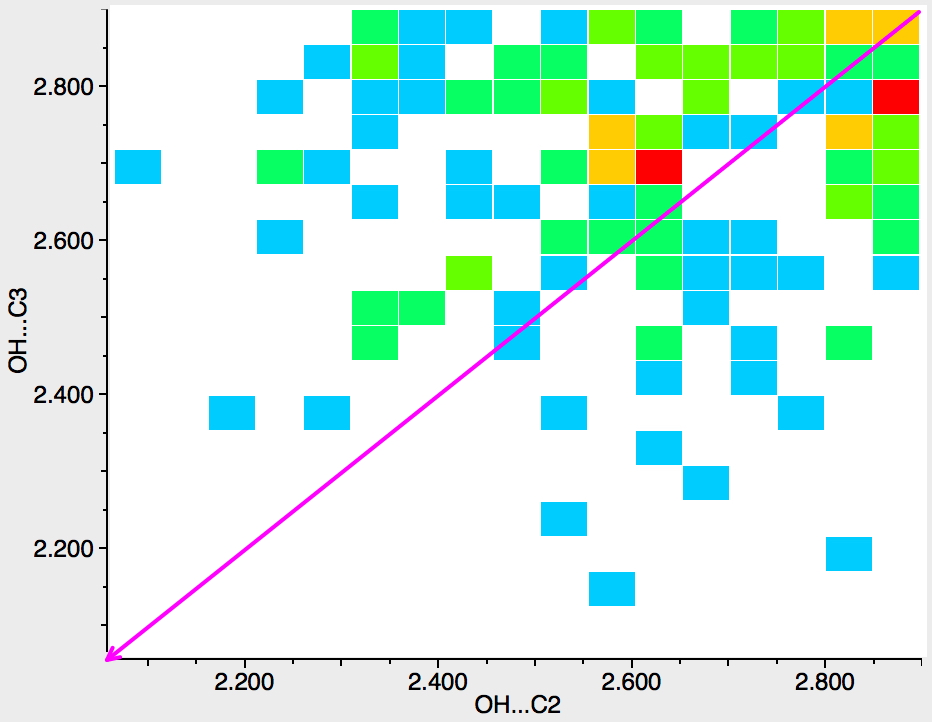
Next, the N atom is replaced by any atom from group 16 of the periodic table (i.e. O, S, etc). The scatter is now in both top left and bottom right triangles, which suggest much weaker discrimination between C2 and C3; if anything in favour of C2 (often the observed regiospecificities for such compounds).
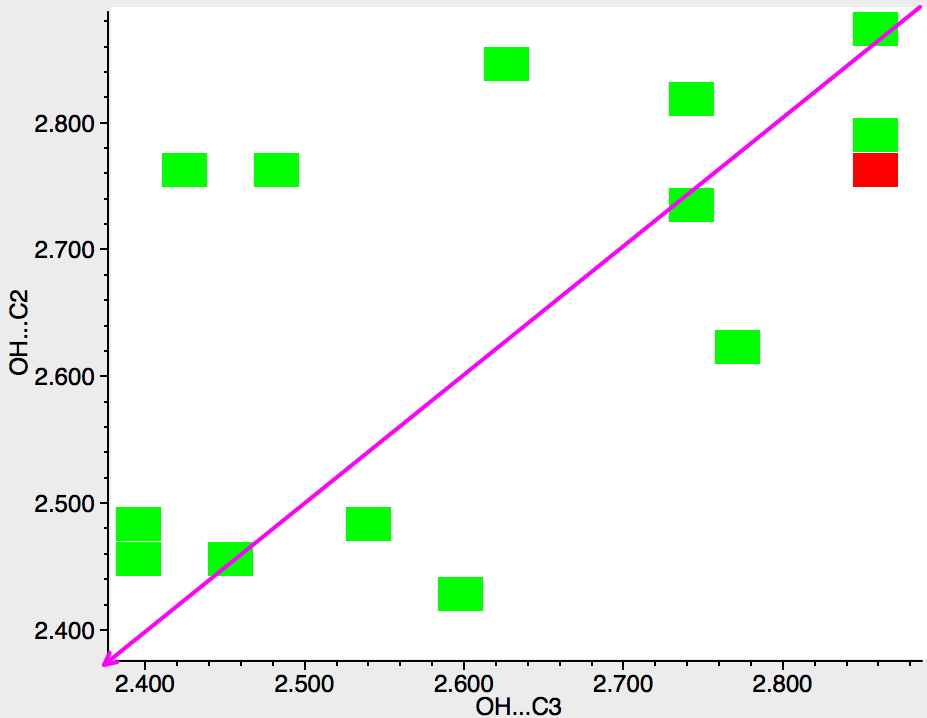
Finally, pyridines. Only a slight bias towards the C2 position. With pyridines of course, the electrophile in fact first interacts with the nitrogen lone pair in the plane of the molecule, which perturbs the eventual outcome. So this crystallographic method is perhaps a better intrinsic probe than kinetic reactivity.
Tags: Asymmetric hydrogenation, benzene, benzo, Electrophile, Furan, Indole, Pyridine, Pyrrole, search query, Simple aromatic rings, Substitution reaction, Thiophene