If H3N+-O– is viable compared with its tautomer H2N-OH when carrying water bridges, then why not try H2O+-O– vs HO-OH?
There are no examples to be found in crystal structures! The solvated structure of H2O+-O– is modified directly from that of H3N+-O– and the computed (ωB97XD/6-311++G(d,p)/SCRF=water) structure[cite]10.14469/ch/192005[/cite] is shown below. Noteworthy is that the hydrogen bonds at the O+ end are far stronger than those to at the O– end.
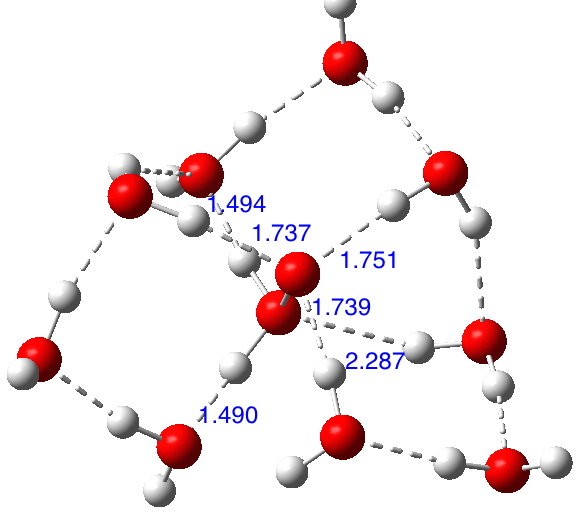
The corresponding hydrated hydrogen peroxide is 16.3 kcal/mol lower in free energy; this compares favourably with the value for water itself and suggests that oxane oxide might also be capable of isolation inside a suitable hydrogen bond stabilising cavity.
Tags: Ammonia, Anions, free energy, Hydrogen bond, Hydrogen peroxide, Inorganic solvents, Oxide, Oxidizing agents, Peroxide, Properties of water