The post on applying VSEPR ("valence shell electron pair repulsion") theory to the geometry of ClF3 has proved perennially popular. So here is a follow-up on another little molecue, F3SN. As the name implies, it is often represented with an S≡N bond. Here I take a look at the conventional analysis.
This is as follows:
- Six valence electrons on the central S atom.
- Three F atoms contribute one electron each.
- One electron from the N σ-bond.
- Donate two electrons from S to the two π-bonds.
- Eight electrons left around central S, ≡ four valence shell electron pairs.
- Hence a tetrahedral geometry.
- The bond-bond repulsions however are not all equal. The SN bond repels the three SF bonds more than the S-F bonds repel each-other.
- Hence the N-S-F angle is greater than the F-S-F angle, a distorted tetrahedron.
Now for a calculation[1]; ωB97XD/Def2-TZVP, where the wavefunction is analysed using ELF (electron localisation function), which is a useful way of locating the centroids of bonds and lone pairs (click on diagram below to see 3D model).
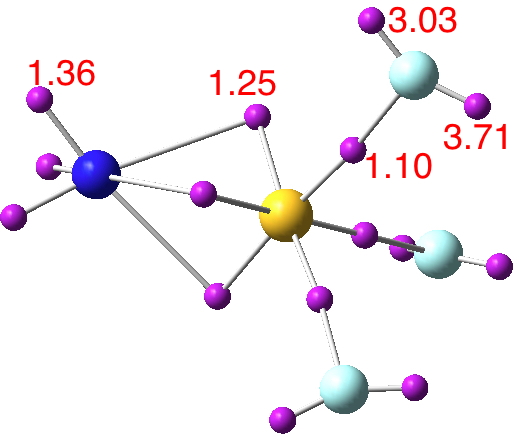
- At the outset one notes that there are six ELF disynaptic basins surrounding the central S, integrating to a total of 7.05e. The sulfur is NOT hypervalent; it does not exceed the octet rule.
- These six "electron sub-pair" basins are arranged octahedrally around the sulfur. The coordination is NOT tetrahedral, as implied above.
- The three S-N basins have slightly more electrons (1.25e) than the three S-F basins (1.10e), resulting in …
- the angle subtended at the S for the SN basins being 96° (a bit larger than octahedral) whilst the angle subtended at the S for the SF basins being smaller (89.9°). This matches point 7 above, but is achieved in an entirely different manner.
- As a result, the N-S-F angle (122.5°) is larger than the ideal tetrahedral angle and the F-S-F angle (93.9°) is smaller, an alternative way of expressing point 7 above.
- The S≡N triple bond as shown above does have some reality; it is a "banana bond" with three connectors rather than two. Each banana bond however has only 1.25e, so the bond order of this motif is ~four (not six) but nevertheless resulting in a short S-N distance (1.406Å) with multiple character.
So we have achieved the same result as classical VSEPR, but using partial rather than full electron pairs to do so. We got the same result with ClF3 before. So perhaps this variation could be called "valence shell partial electron pair repulsions" or VSPEPR.
References
- Henry S Rzepa., "F 3 N 1 S 1", 2016. http://dx.doi.org/10.14469/ch/191808
Tags: Chemical bond, chemical bonding, Electron, Hypervalency, Lone pair, Molecular geometry, Octet rule, Quantum chemistry, Stereochemistry, Tetrahedral molecular geometry, Theoretical chemistry, Valence, VSEPR theory