Here is another exploration of simple chemical concepts using crystal structures. Consider a simple diene: how does the central C-C bond length respond to the torsion angle between the two C=C bonds?
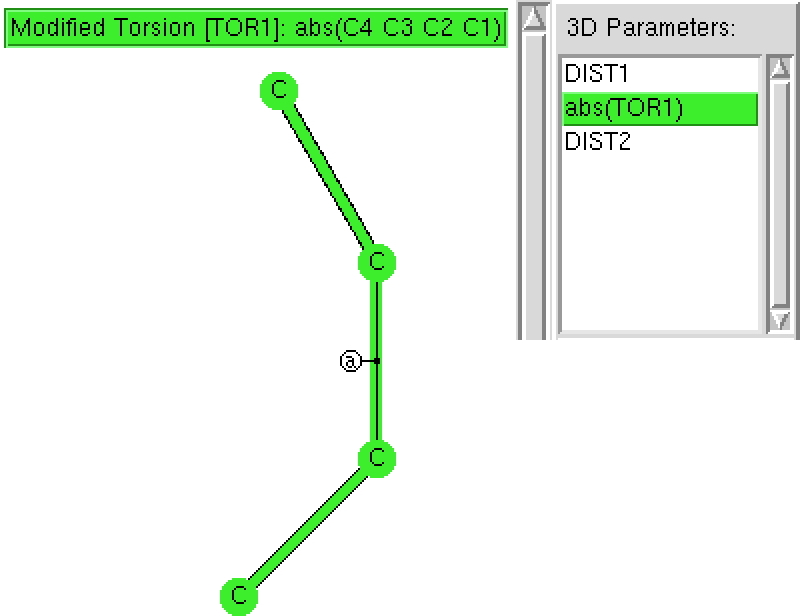
The search of the CSD (Cambridge structure database) is constrained to R < 5%, no errors and no disorder and the central C-C bond is specific to be acyclic.‡
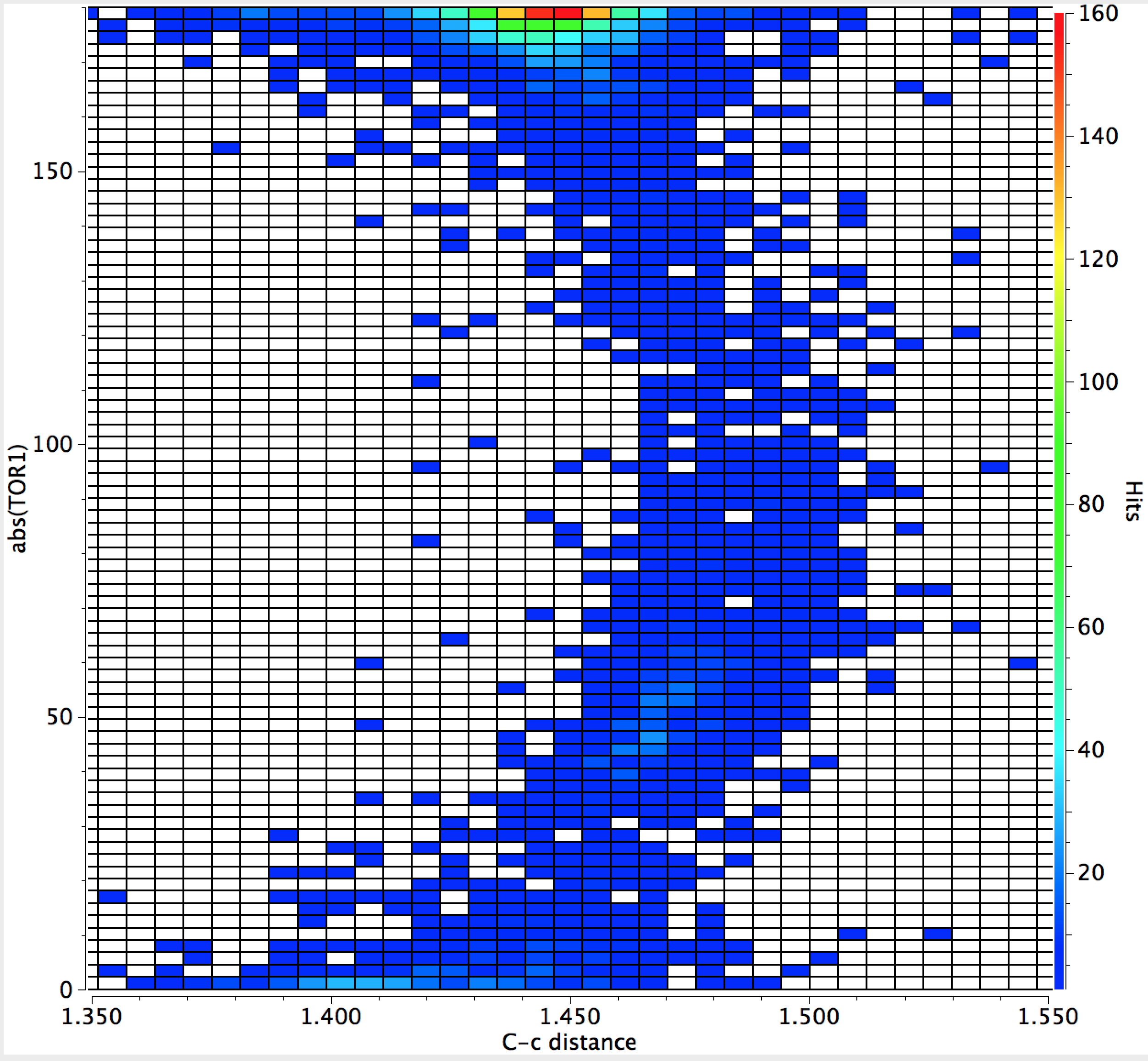
- Note first that the hotspot occurs for a torsion angle of 180°, a trans diene.
- There is just a hint that the C-C distance for a cis-diene might be a little shorter than the trans diene, but this might not be significant.
- There is a gentle curve illustrating that the C-C distance is indeed a maximum at 90°
- The C-C bond extends from ~1.445Å when the two double bonds are coplanar (fully conjugated) to ~1.48Å when orthogonal. Not much of a change, but statistically highly significant.
Here is another search, this time of the C=C-C=C motif embedded into a biaryl, of which there are far more examples. This time, the (red) hotspot is actually at 90°, with local (green) hotspots at 0 and 180° but also at 45 and 135°. Again, you can easily spot the maximum in C-C bond length at 90° but notice how much smaller the bond lengthening is (~ 0.01Å). This lengthening is inhibited by retention of the aromaticity of the two aryl rings; again the statistical effect is highly significant. Perhaps also significant is that the C-C bond at torsions of 0 or 180° appear to be no shorter than the values at 45 and 135°.
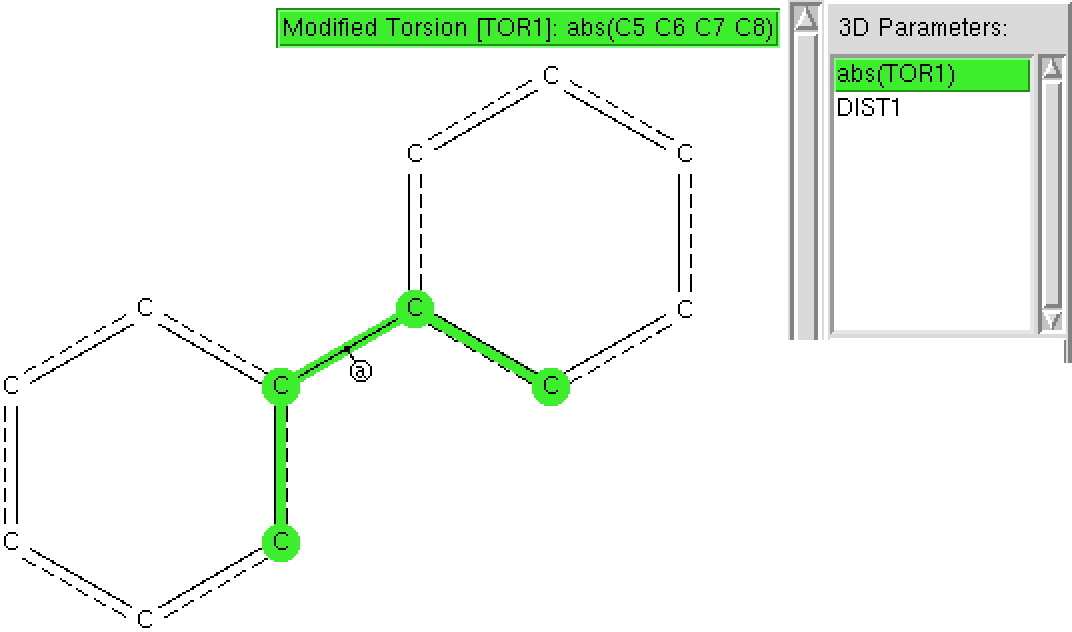
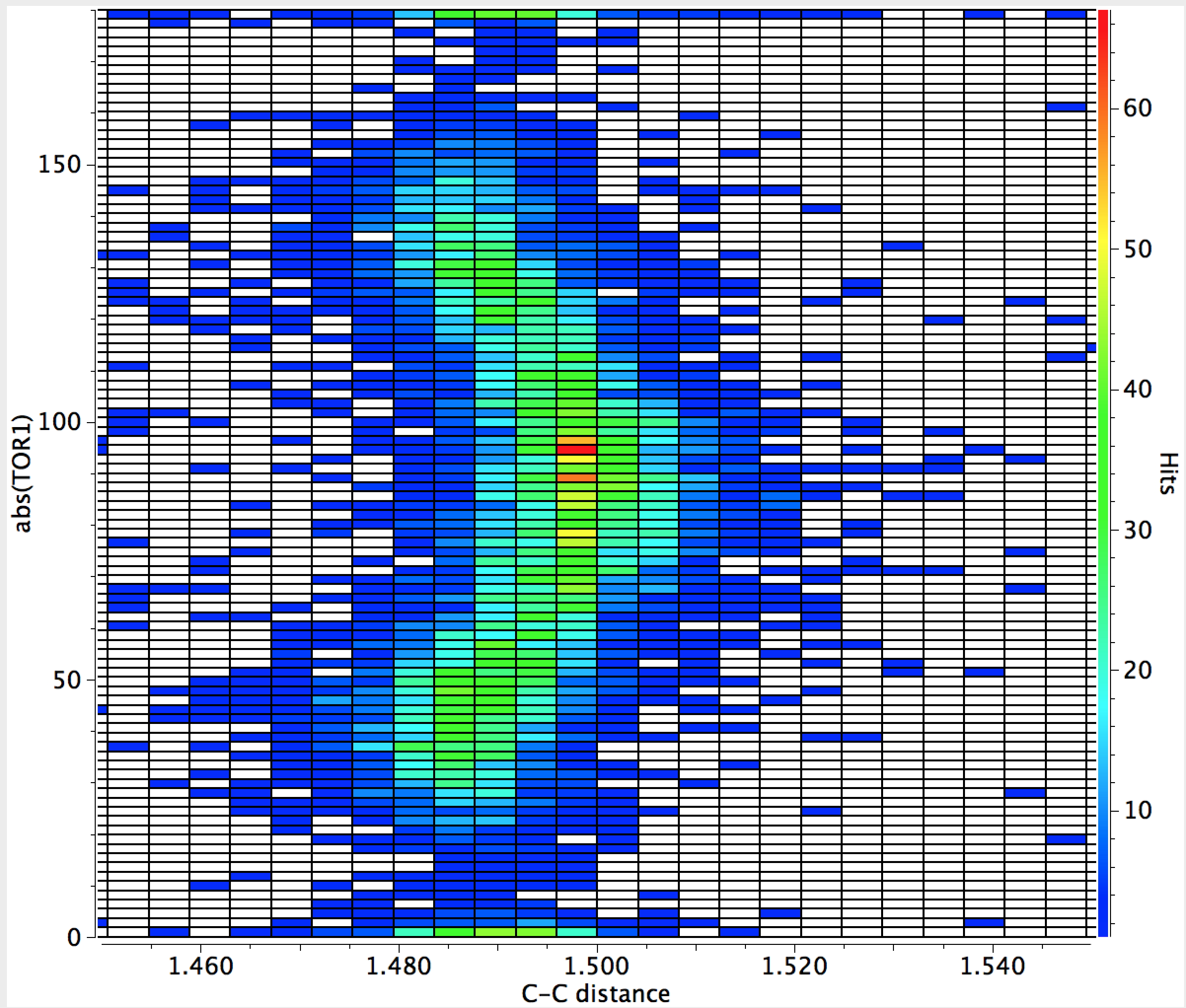
Both these searches took about 5 minutes each, and serve to illustrate just how many basic chemical concepts can be teased out of a statistical analysis of crystal structures.
‡The analogous diagram for O=C-C=C is shown below;
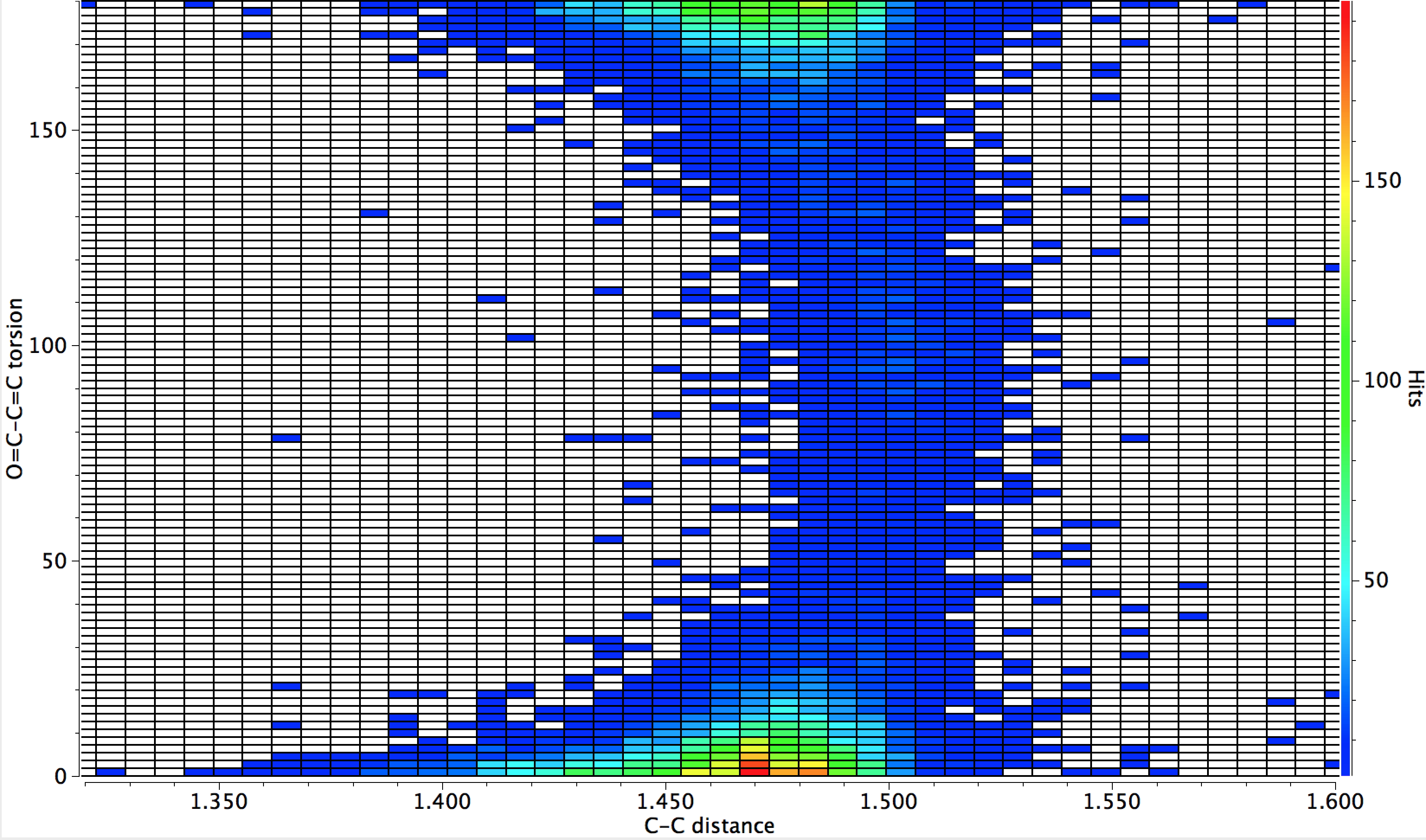
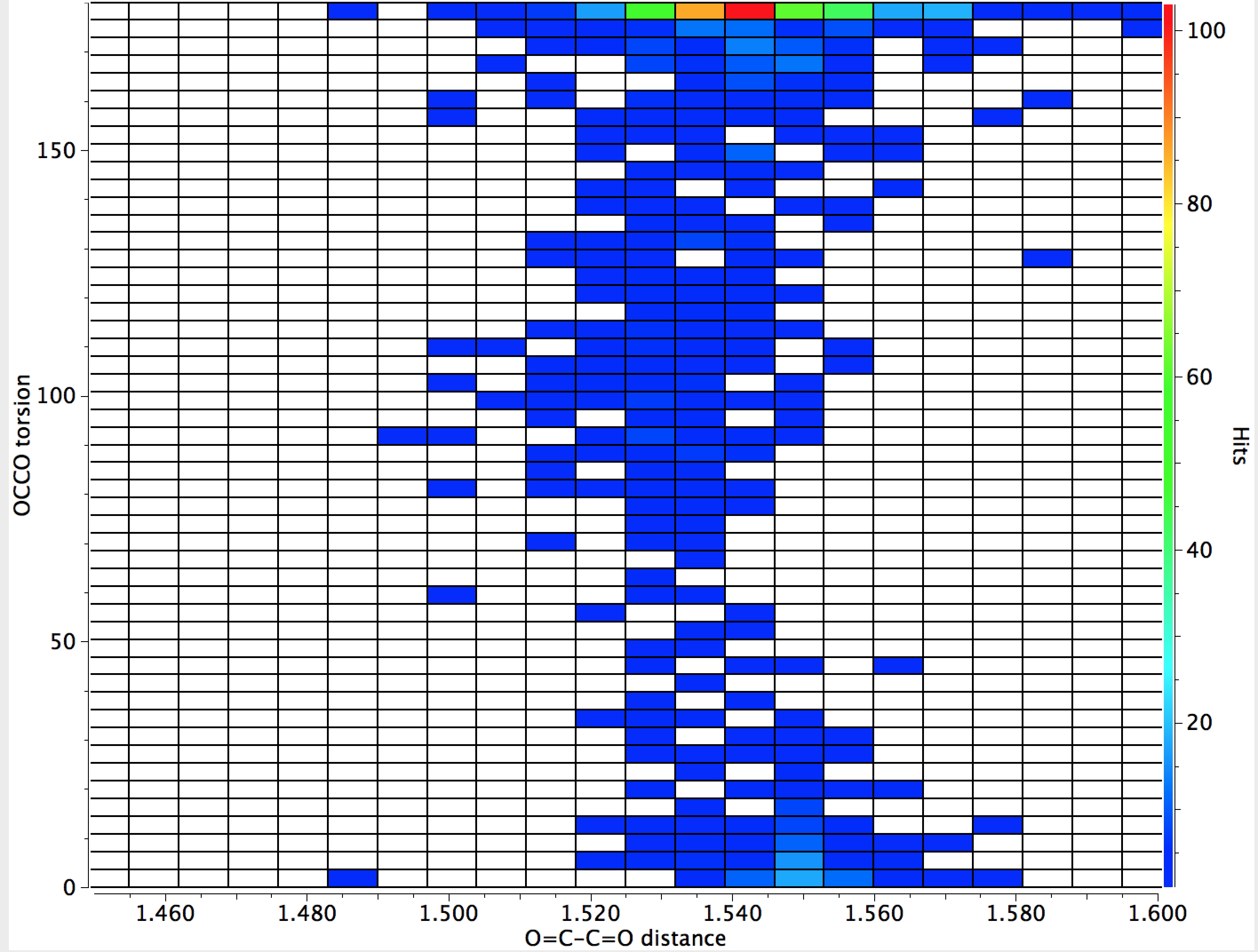
That for O=C-C=O is different however;
Acknowledgments
This post has been cross-posted in PDF format at Authorea.
Tags: basic chemical concepts, Cambridge, Chemical bond, chemical concepts using crystal structures, Chemical IT, Quantum chemistry