Peter Edwards has just given the 2015 Hofmann lecture here at Imperial on the topic of solvated electrons. An organic chemist knows this species as “e–” and it occurs in ionic compounds known as electrides; chloride = the negative anion of a chlorine atom, hence electride = the negative anion of an electron. It struck me how very odd these molecules are and so I thought I might share here some properties I computed after the lecture for a specific electride known as GAVKIS.[cite]10.1107/S0108270188002847[/cite] If you really want to learn (almost) everything about these strange species, go read the wonderful review by Zurek, Edwards and Hoffmann,[cite]10.1002/anie.200900373[/cite] including a lesson in the history of chemistry stretching back almost 200 years.
GAVKIS consists of a tricyclic aza-ether ligand or cryptand wrapping a potassium atom in the centre, the overall unit having no charge. The oxygen and nitrogen heteroatoms coordinate to the metal, in the process evicting its single electron. The question that struck me is “where does that electron go?”. You see in all normal molecules that electrons are associated with either one, two (or rarely) three nuclei, to form one-centred monosynaptic basins (lone pairs), two-centre or disynaptic basins (i.e. bonds) and more rarely three-centre bonds. The shared-electron two-centre manifestation was of course famously introduced by Gilbert N. Lewis in 1916 (note the centenary coming up!). Knowing where the electron (pairs) are has enabled the technique popular with organic chemists known as arrow pushing, or the VSEPR analysis of inorganic compounds. But an electride has no nucleus associated with it! So how can one describe its location?
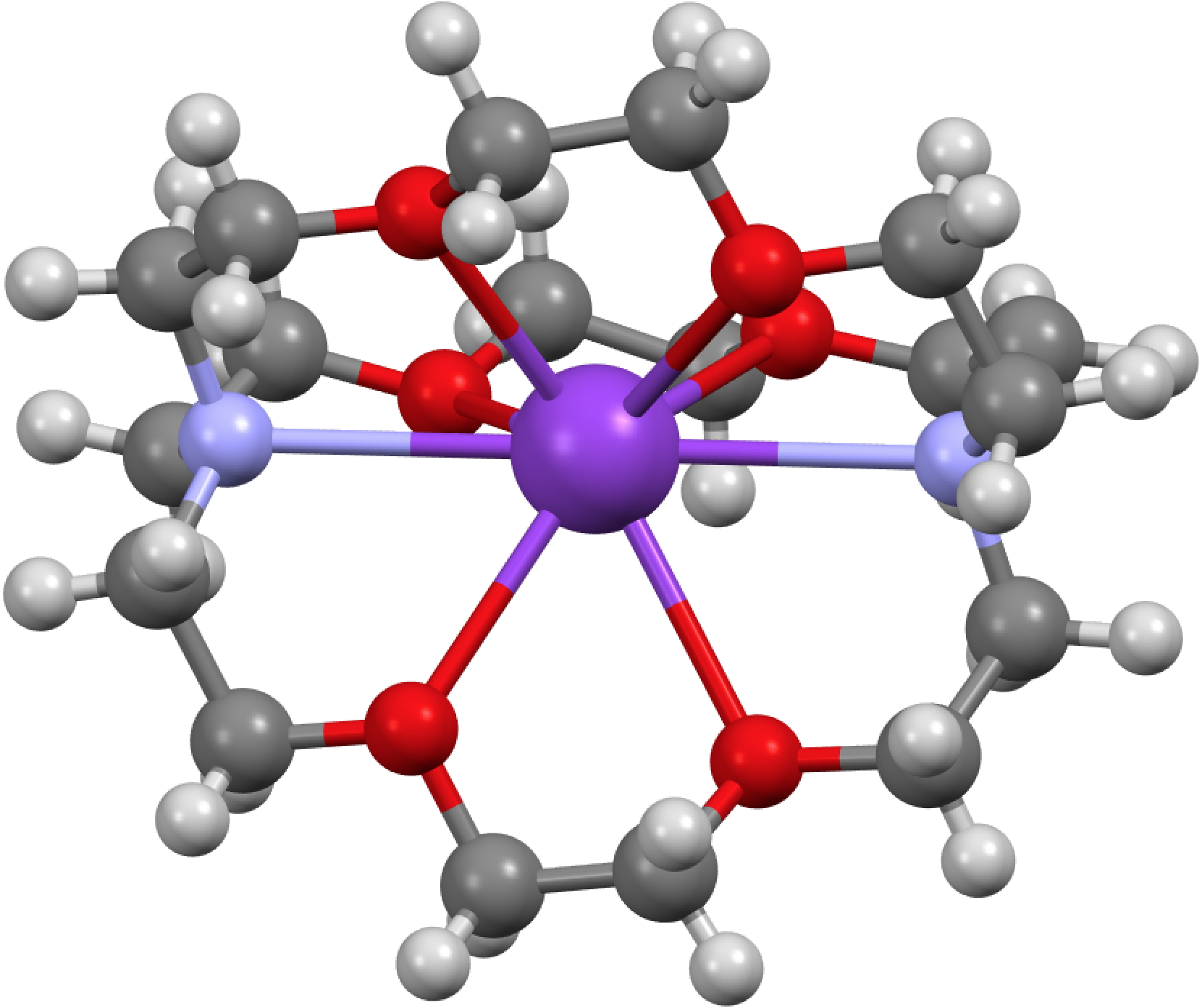
Click for 3D structure of GAVFIS
The crystal structure of GAVFIS shows the potassium to be 8-coordinate. Remember, x-rays are diffracted not by a nucleus but by electrons in the molecule. The highest densities are of course associated with electrons in inner shells centered on nuclei and the much lower densities found in conventional bonds are not normally located by this technique (but see here). So it is no surprise to find that this x-ray analysis[cite]10.1107/S0108270188002847[/cite] did not succeed in answering the question posed above; where is the single electron liberated from the potassium atom? They did look for it, but surmised only that would be found in the “noise level electron density in the spaces between them (molecules)“. For GAVFIS, that empty space is actually dumb-bell shaped, and so perhaps an answer is that the electron occupies the dumb-bell shaped spaces between the ligand-potassium complex.
X-ray analysis was defeated by noise; it is an experimental technique after all. But the noise in a quantum mechanical calculation is much smaller; can this reveal where the evicted electron is? Here is the spin density (unpaired electron) distribution for one molecule of GAVFIS computed using the UωB97XD/6-31++(G) DFT method.[cite]10.14469/ch/191347[/cite].[cite]10.14469/ch/191354[/cite] It is a stratocumulus-like cloud that enshrouds the molecule (click on the diagram below and you can rotate the function to view it from your own point of interest) but interestingly avoiding the regions along the N….N axis. There are also tiny amounts of (negative) spin density on the ligand atoms. So even when the “empty space” is infinitely large, the shape of the electride anion is nevertheless quite specific, but a holistic function of the shape of the entire molecule rather than its component atoms.‡

Click for 3D
Another way of describing where electrons are is using functions known as molecular orbitals. Below is the SOMO (singly occupied MO) and its shape in this case coincides with that of the spin density.

Click for 3D interaction
The molecular electrostatic potential is rather wackier (red = attractive to protons).

Click for 3D interaction
Odder still is the ELF (electron localisation function) and the identification of the centroids of its basins. These centroids normally coincide with the two-centre basins (bonds) and one-centre basins (lone pairs, inner shell electrons) in normal molecules, both being close to nuclear centres (atoms). For GAVFIS, two unexpected one-centre basins are found close to the two nitrogen atoms in the molecule, each with a population of 0.48 electrons, along with regular one-centre “lone pair” basins pointing inwards to the potassium (2.38 electrons each). The odd-looking pair of locations identified for the electride anion may have little physical reality, except for reminding us that the electride can indeed be in more than one location simultaneously!
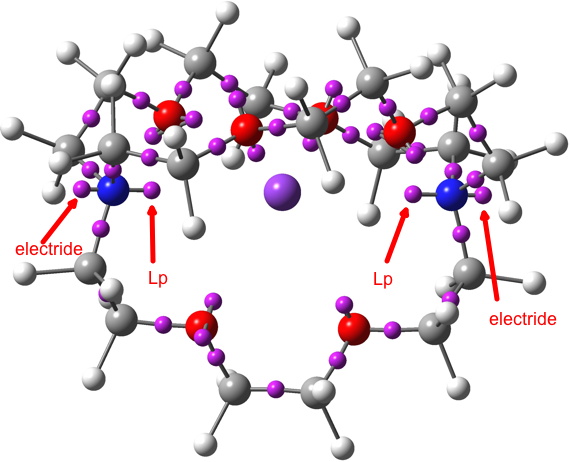
I often also use the NCI (non-covalent-interaction) property of the electron density in these blogs. It tells us about regions of non-covalent electron density which represent attractive weak interactions between or within molecules. Here, it again shows us the weak non-covalent density (as the reduced density gradients) wrapping the molecule (green=weakly stabilizing).

Click for 3D interaction
The obvious next question is that if each molecule is surrounded by weak spin density arising from an unpaired electron, would two such species form a dimer in which the spins are paired in an manner analogous to the conventional single bond? The overlap is not going to be fantastic if the spin distribution has the shape shown above, but what the hell. Here is the HOMO of such a species.[cite]10.14469/ch/191348[/cite] It appears the shape of the electride is very pliable indeed; they have been squeezed out of the contact region between the two molecules (which form a close contact pair) into wrapping the dimer rather than the monomer! The spin-coupled singlet by the way is about 4.6 kcal/mol more stable in free energy ΔG298 than two isolated monomer doublets, and 5.5 kcal/mol lower than the triplet species[cite]10.14469/ch/191350[/cite] which retains two unpaired electrons. A sort of weak molecule-pair bond rather than an atom-pair bond.

Click for 3D interaction
This has hardly started to scratch the surface of the strange properties of electrides. If your appetite has been whetted, go read the article I noted at the beginning.[cite]10.1002/anie.200900373[/cite]
‡ For normal molecules, a Mulliken or other population analysis reduces the charge and spin density down to an atom-centered distribution. If this is done for GAVFIS, the spin density collapses down to the molecular centroid, in this case the potassium (spin density 1.15). This of course is horribly misleading, and serves to remind us that such atom-centered distributions can sometimes be far from realistic.