I do not play poker,‡ and so I had to look up a 5-4-3-2-1(A), which Wikipedia informs me is a 5-high straight flush, also apparently known as a steel wheel. In previous posts I have suggested acids which can be ionised by (probably) 5, 4, 3 or 1 discrete water molecules in the gas phase; now to try to track down a candidate for ionisation by the required two water molecules to form that straight flush.
As the counter-anion to quaternary ammonium cations, bis(trifluoromethylsulfonyl)imide is a component of some ionic liquids. Its conjugate acid is thought[1],[2] to protonate on the nitrogen.

Click for 3D
My first obvious attempt was to place two waters near that N-H to see if it would ionise from that position.[3] The proton remains attached to the nitrogen(-:
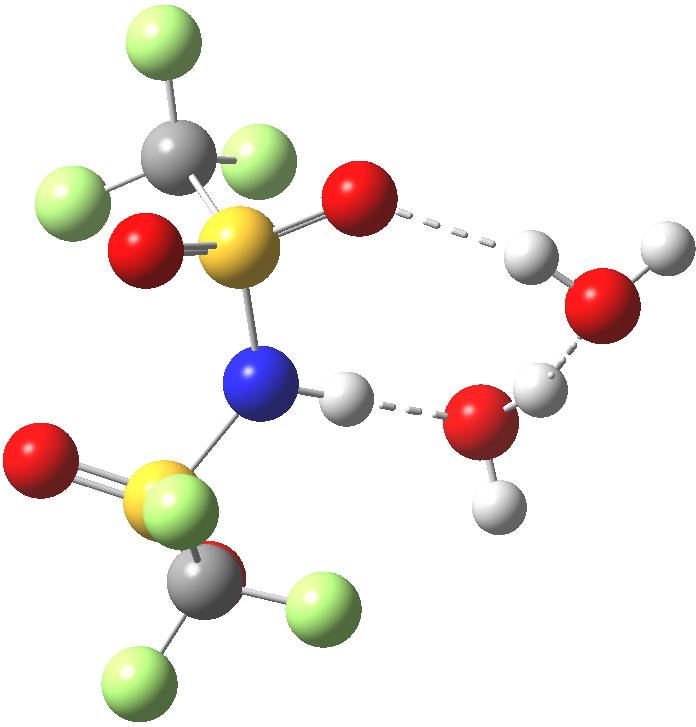
Next, how about re-locating the waters so that they are closer to the sulfonyl oxygens? This time we do have the characteristic hydronium cation forming.[4] However, the free energy of this isomer is +6.7 kcal/mol higher relative to the NH form. So not a 5-high straight flush in a strict sense, but it perhaps does give a hint of how one might design the missing card.

Click for 3D
‡Confession time. I did spend many a Wednesday afternoon as an undergraduate playing the card game bridge.
References
- "Structures of bis(fluorosulfonyl)imide HN(SO2F)2, bis(trifluoromethylsulfonyl)imide HN(SO2CF3)2, and their potassium salts at 150 K", Zeitschrift für Kristallographie - Crystalline Materials, vol. 213, pp. 217-222, 1998. http://dx.doi.org/10.1524/zkri.1998.213.4.217
- Zak, Z.., and Ruzicka, A.., "CCDC 119129: Experimental Crystal Structure Determination", 1999. http://dx.doi.org/10.5517/CC3ZYWW
- Henry S Rzepa., "C 2 H 5 F 6 N 1 O 6 S 2", 2015. http://dx.doi.org/10.14469/ch/191136
- Henry S Rzepa., "C 2 H 5 F 6 N 1 O 6 S 2", 2015. http://dx.doi.org/10.14469/ch/191137
Tags: free energy, gas phase, Interesting chemistry, steel wheel