HCN is a weak acid (pKa +9.2, weaker than e.g. HF), although it does have an isomer, isocyanic acid or HNC (pka < +9.2 ?) which is simultaneously stronger and less stable. I conclude my halide acid series by investigating how many water molecules (in gas phase clusters) are required for ionisation of this “pseudo-halogen” acid.
First some observations about the structures of these complexes. The negative charge that develops on the cyanide is no longer atom-centered but is delocalised across two atoms, and furthermore the C≡N triple bond is also quite basic. So the stabilising hydrogen bonds have more choices than with the halide anions. To characterise these weak interactions, I show the structures here with the NCI (non-covalent-interaction) function included.[1] The colour coding is blue=strong attraction, green=weaker attraction, yellow=weak repulsions.
- The 2H2O complexes have insufficient length to bridge across from the H to the developing charge on the cyanide. Hence instead you see weaker π-facial interactions. Contrast also the dark blue of the NCI interaction to NH but the lighter cyan to CH.
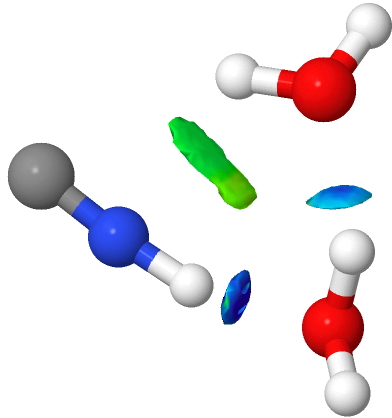
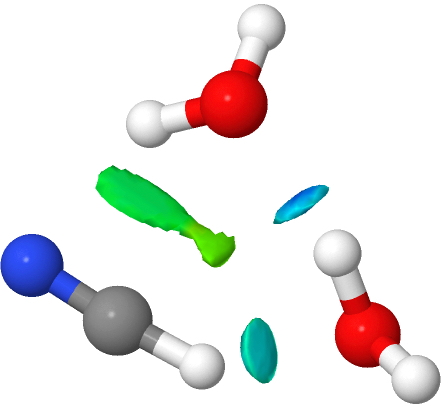
- The 3H2O complexes abandon the weaker π-facial interactions to form a more normal H-bond to the terminal atom of the cyanide. The angle is far from optimal, and the colour coding reflects this weakening (cyan rather than deep blue). Note the small green zone in the middle of the ring, a residual π-facial mode.
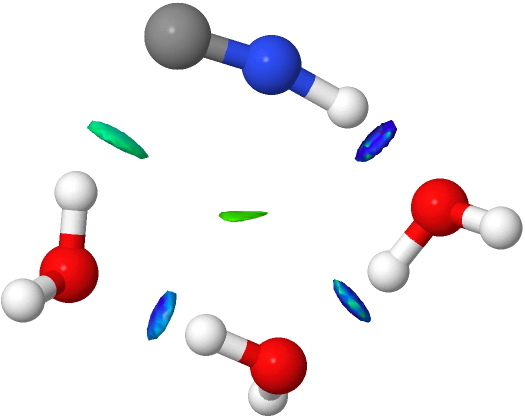
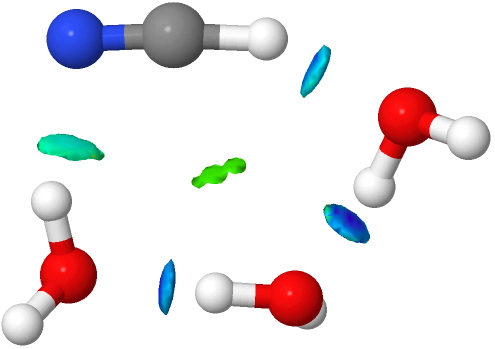
- Here we see conventional H-bonds, but note now the deep blue NCI for the CH…O interaction, and the cyan for the OH…N for the first example.
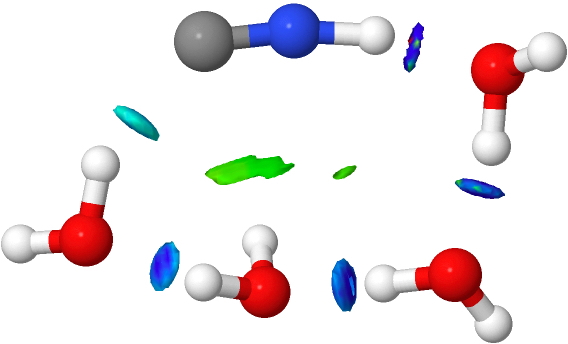
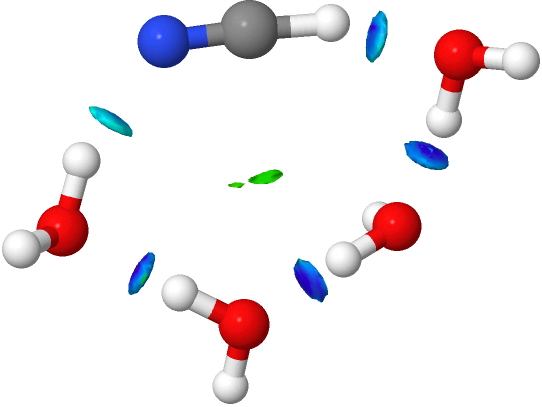
- An interesting new feature appears with five water molecules. Lots of blue-coloured NCI, but one is missing (red arrow). This is because NCI is filtered to remove electron densities above a specified threshold, since these are no longer deemed “non-covalent” but start to fall into the covalent regions. The hydrogen bond specified by the red arrow is such.
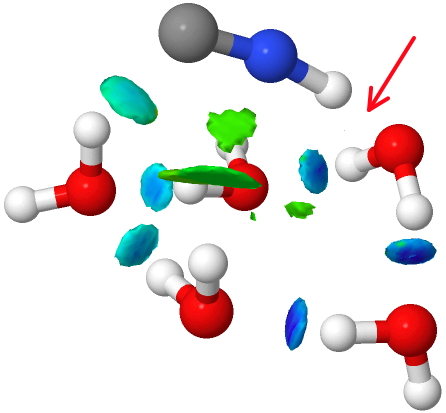
If that density threshold is raised (0.07 au), the deep blue feature can now be seen. So we have the concept here that a hydrogen bond can indeed be too strong to be “non-covalent” and it passes the (rather arbitrary) threshold into being covalent. A new way of classifying hydrogen bonds!
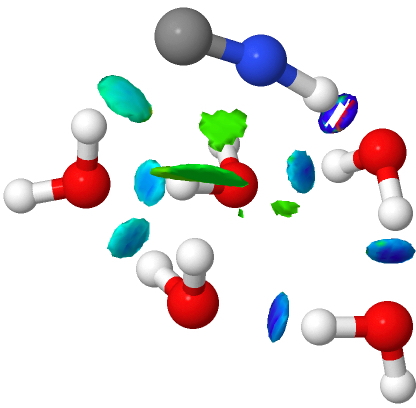
The surprises are not quite over yet. Below is an isomer in which the water arrangement is different. This is much more ionic, as shown by three regions of covalent hydrogen bonds (red arrows) and a fully ionised cyanide supporting two hydrogen bonds to it, not one as before. The free energy of this alternative however is 5.1 kcal mol-1 higher than the previous non-ionic form.
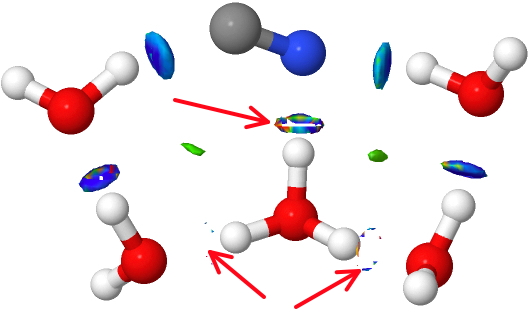
With the HCN isomer, the normal thresholds again apply.
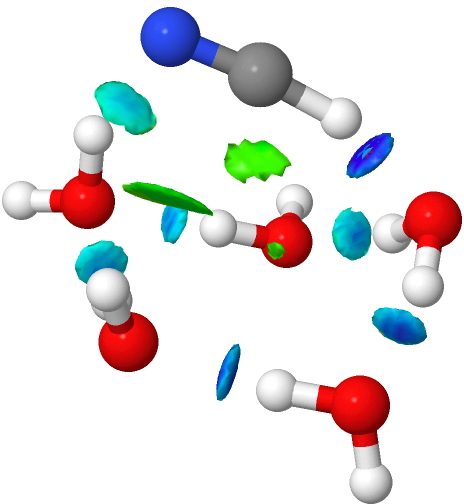
- With six waters and HNC, ionisation now occurs and the NCI feature appears in the N…H region rather than the H…O. The ionic nature also manifests with four other H…O regions (red arrows) where the non-covalent NCI threshold is passed or almost passed, and the covalent one starts.
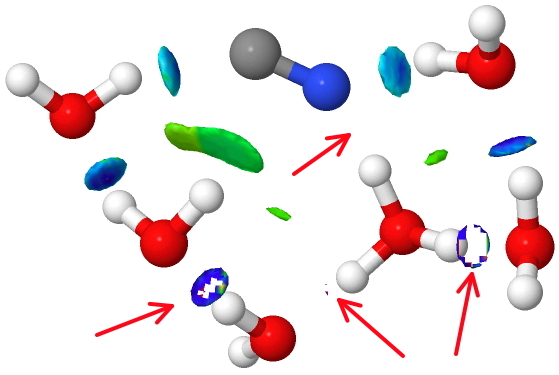
Note how much green dispersion attraction is starting to appear in this caged structure and how strong (NCI = deep blue) the C-H…O hydrogen bond has become (CH hydrogen bonds in ionic systems are indeed much stronger than they are given credit for).
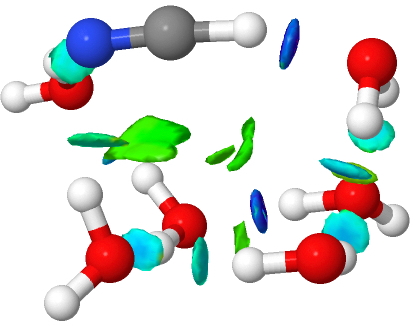
The transition to ionicity can also be seen with the trend bond lengths, and the sudden discontinuity with six water molecules.
| HCN | HNC | |||
|---|---|---|---|---|
| n | C-H | H-O | N-H | H-O |
| 1 | 1.077 | 2.021[2] | 1.015 | 1.806[3] |
| 2 | 1.078 | 2.064[4] | 1.027 | 1.736[5] |
| 3 | 1.086 | 1.913[6] | 1.037 | 1.667[7] |
| 4 | 1.089 | 1.864[8] | 1.042 | 1.637[9] |
| 5 | 1.103 | 1.795[10] | 1.074 1.609 |
1.522[11] 1.021[12] |
| 6 | 1.106 | 1.767[13] | 1.525 | 1.041[14] |
To summarise, HNC is a relatively strong acid, and six water molecules are required to ionise it. In contrast, HCN is much weaker and so it is not ionised even by six waters, much like HF.
Acknowledegments
This post has been cross-posted in PDF format at Authorea.
References
- A. Armstrong, R.A. Boto, P. Dingwall, J. Contreras-García, M.J. Harvey, N.J. Mason, and H.S. Rzepa, "The Houk–List transition states for organocatalytic mechanisms revisited", Chem. Sci., vol. 5, pp. 2057-2071, 2014. http://dx.doi.org/10.1039/C3SC53416B
- Henry S Rzepa., "C 1 H 3 N 1 O 1", 2015. http://dx.doi.org/10.14469/ch/190936
- Henry S Rzepa., "C 1 H 3 N 1 O 1", 2015. http://dx.doi.org/10.14469/ch/190935
- Henry S Rzepa., "C 1 H 5 N 1 O 2", 2015. http://dx.doi.org/10.14469/ch/190940
- Henry S Rzepa., "C 1 H 5 N 1 O 2", 2015. http://dx.doi.org/10.14469/ch/190937
- Henry S Rzepa., "C 1 H 7 N 1 O 3", 2015. http://dx.doi.org/10.14469/ch/190938
- Henry S Rzepa., "C 1 H 7 N 1 O 3", 2015. http://dx.doi.org/10.14469/ch/190939
- Henry S Rzepa., "C 1 H 9 N 1 O 4", 2015. http://dx.doi.org/10.14469/ch/190951
- Henry S Rzepa., "C 1 H 9 N 1 O 4", 2015. http://dx.doi.org/10.14469/ch/190954
- Henry S Rzepa., "C 1 H 11 N 1 O 5", 2015. http://dx.doi.org/10.14469/ch/190964
- Henry S Rzepa., "C 1 H 11 N 1 O 5", 2015. http://dx.doi.org/10.14469/ch/190957
- Henry S Rzepa., "C 1 H 11 N 1 O 5", 2015. http://dx.doi.org/10.14469/ch/190968
- Henry S Rzepa., "C 1 H 13 N 1 O 6", 2015. http://dx.doi.org/10.14469/ch/190971
- Henry S Rzepa., "C 1 H 13 N 1 O 6", 2015. http://dx.doi.org/10.14469/ch/190965