The concept of a “hidden intermediate” in a reaction pathway has been promoted by Dieter Cremer[1] and much invoked on this blog. When I used this term in a recent article of ours[2], a referee tried to object, saying it was not in common use in chemistry. The term clearly has an image problem. A colleague recently sent me an article to read (thanks Chris!) about isotope effects in the epoxidation of ethene[3] and there I discovered a nice example of hidden intermediates which I share with you now.
The reaction above is considered as a transfer of an oxygen atom to an alkene with concomitant concerted proton transfer (for the analogous reaction of an alkyne see here). The mechanism is normally illustrated with five arrows accomplishing both operations (see below). But a simple experiment shows this cannot be accurate[3]. When d4-ethene is used (with mCPBA as the peracid) in dichloromethane solution, an inverse kinetic isotope effect of 0.83 is observed (in other words the d4– species reacts faster than the 1H). If the peracid is instead deuterated (as OD) the reaction slows down by a factor of 1.05 (a normal isotope effect). This data provides a good test of whether any transition state model constructed for the reaction is a good one. So how about a ωB97XD/6-311G(d,p)/SCRF=dichloromethane[4],[5] model, using per-ethanoic acid? The isotope effect can be obtained by calculating the activation free energy with the appropriate isotope specified.
| Value | Normal isotopes | d4-ethene | OD acid | One 13C-ethene | One 18O |
| ΔG298 | 26.6844375 | 26.5589375 | 26.7245975 | 26.6907125 | 26.7296175 |
| KIE | – | 0.808 | 1.070 | 1.011 | 1.080 |
The deuterium isotope effects, on both carbon and oxygen compare very well indeed with those measured (the 13C and 18O have never been measured, they are predictions), which greatly assures that the model is a good one. So the predicted transition state geometry as shown below has had a good reality check. It reveals that two O-C bonds are forming, and the O-O bond is breaking, but that proton transfer has hardly started. This corresponds to the three green arrows shown at the top. The three blue arrows are not yet in action. I have colour-coded them to illustrate this temporal aspect.
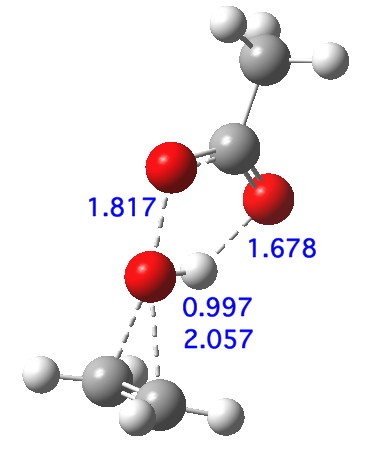
An intrinsic reaction coordinate[6] shows that the reaction is concerted, albeit asynchronous; the transition state (TS, @IRC=0.0) really only involves the green arrows; the blue arrows kick in only AFTER the ion-pair-like hidden intermediate is formed, labelled HI above (and seen @IRC =+1.2). Stabilising the ion-pair using trifluorethanoic acid[7] (a stronger acid) renders the HI more prominent.
| – |
As quantum calculations apparently give us trustworthy indications of the order in which events happen during this reaction mechanism, perhaps it is time to start representing this information in our simple schematics. (Some) text-books currently show the left hand diagram below, involving five arrows but with no indication of their relative timing. The right hand side now colours the arrows to indicate that the green precede the blue. An extra arrow is added to indicate that one electron pair is involved in forming a “hidden intermediate”, the green arrow aspiring to end at a lone pair, but the blue arrow then continuing its progress to the final epoxide. The end-point of one arrow representing the start point of another could thus be taken as implying a hidden intermediate.
As I have noted elsewhere, the curly-arrow representation of reaction mechanism has hardly evolved over the last sixty years. Some might argue that such stability is appropriate for a very simple heuristic used to teach introductory chemistry to students, and that this very simplicity should not be gratuitously discarded. But perhaps, in the light of what we now know about many mechanisms, it has become over-simple? Could judiciously deployed colour-coding‡ of the arrows be a useful, albeit perhaps only a small step, to upgrading arrow pushing into the 21st century?
‡I appreciate that some people are red-green/blue-yellow colour-blind, and that a full ROYGBIV spectrum of colours may carry too much information!
References
- E. Kraka, and D. Cremer, "Computational Analysis of the Mechanism of Chemical Reactions in Terms of Reaction Phases: Hidden Intermediates and Hidden Transition States", Accounts of Chemical Research, vol. 43, pp. 591-601, 2010. http://dx.doi.org/10.1021/ar900013p
- H.S. Rzepa, and C. Wentrup, "Mechanistic Diversity in Thermal Fragmentation Reactions: A Computational Exploration of CO and CO2 Extrusions from Five-Membered Rings", The Journal of Organic Chemistry, vol. 78, pp. 7565-7574, 2013. http://dx.doi.org/10.1021/jo401146k
- T. Koerner, H. Slebocka-Tilk, and R.S. Brown, "Experimental Investigation of the Primary and Secondary Deuterium Kinetic Isotope Effects for Epoxidation of Alkenes and Ethylene with m-Chloroperoxybenzoic Acid", The Journal of Organic Chemistry, vol. 64, pp. 196-201, 1998. http://dx.doi.org/10.1021/jo981652x
- Henry S. Rzepa., "Gaussian Job Archive for C4H8O3", 2013. http://dx.doi.org/10.6084/m9.figshare.781238
- Henry S. Rzepa., "Gaussian Job Archive for C4H8O3", 2013. http://dx.doi.org/10.6084/m9.figshare.781284
- Henry S. Rzepa., "Gaussian Job Archive for C4H5F3O3", 2013. http://dx.doi.org/10.6084/m9.figshare.781283
Tags: activation free energy, arrow pushing, curly arrow, dichloromethane solution, Dieter Cremer, hidden intermediate, model, using per-ethanoic acid
For completeness, I have included the KIE evaluated for m-chloroperbenzoic acid as oxidant, and have applied a scaling factor of 0.9 to the frequencies (to approximately correct for anharmonicity and other errors).
The data citations are: 10.6084/m9.figshare.782244 (reactant) and 10.6084/m9.figshare.781326 (transition state). If you wish to use these to e.g. calculate other isotope effects, download the
.fchkfile, convert it to a.chkfile using the utilityunfchk, and re-run the free energy calculation by first reading in the force constant matrix and geometry from the checkpoint file, and then specifying the isotopes you wish to calculate (and the temperature and scaling factor). This is a particularly good example, I think, of the value of citing sharable data.[…] Chemistry with a twist « Experimental evidence for “hidden intermediates”? Epoxidation of ethene by peracid. […]
For the 13C isotope effects and out comparison of experiment and prediction, from 1997:
https://pubs.acs.org/doi/10.1021/ja963656u