I recently got an email from a student asking about the best way of rationalising epoxide ring opening using some form of molecule orbitals. This reminded me of the famous experiment involving propene epoxide.[cite]10.1021/ja01208a047[/cite]
In the presence of 0.3% NaOH, propene epoxide reacts with ethanol at the unsubstituted carbon (~82% compared with 56% in pure water, with retention of configuration at the other carbon) but in 1.3% H2SO4, the predominant product involves attack of ethanol at the more substituted carbon (presumably with inversion of configuration at that carbon). There are many ways of modelling this, but here I choose a simple one; inspecting the energy of the lowest unoccupied orbital (the one that will interact with the lone pair orbital on the incoming nucleophile). The issue is what kind of orbital that should be? The best to choose in this sort of situation is a localized orbital, an NBO in fact.
| NaOH | H2SO4 |
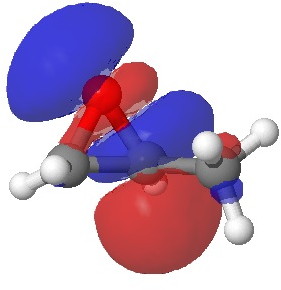 Energy 0.353 au |
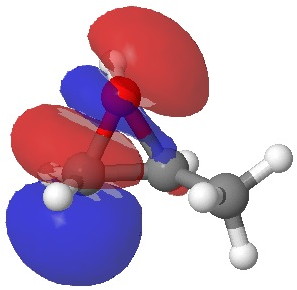 Energy -0.004 au
|
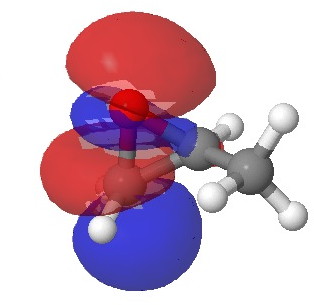 Energy 0.347 au |
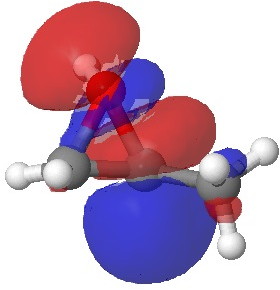 Energy -0.061 au |
In NaOH solutions (where protonation of the epoxide oxygen is suppressed), the lowest energy unoccupied NBO is the O-C σ* orbital involving the unsubstituted carbon, but where the oxygen is protonated, it now becomes the O-C σ* orbital involving the substituted carbon.
One can also teach a simpler heuristic, namely that protonation of the epoxide encourages the early formation of a carbocation, and that the most substituted such cation is the most stable. In the absence of protonation, some (small) contribution from an incipient alkoxy anion favours the alkoxide formed on the more stable carbon.
Tags: 10.1021, energy, lowest energy, predominant product, Reaction Mechanism, Tutorial material