The mechanism of ester hydrolysis is a staple of examination questions in organic chemistry. To get a good grade, one might have to reproduce something like the below. Here, I subject that answer to a reality check.
In this scheme, HA is a general acid, R=Me, and the net result is to break what is called the acyl-oxygen bond (red). The mechanism is actually incomplete, since the label PT designates a proton-transfer (the mechanism for which is left somewhat undefined). Additionally, a lot of charges come and go and five steps or so are involved. So a student might be tempted to “fast-track” the whole process. Below I show two such fast-tracks (I prefer to say simplifications):
In the blue mechanism, the role of HA is actually played by one water molecule, and a second water is assisting the PT step (a far more thorough analysis of the mechanism can be found in this reference[cite]10.1139/V09-011[/cite]). The reaction is bimolecular in ester and the HA (=water in this case). The third water would make it a termolecular reaction overall, but if the reaction takes place in water itself than [H2O] would be constant. It would correspond to what the text books call AAC2 since we consider one molecule as an acid HA. But, one could look at it differently and consider the second water as a nucleophile generated by concurrent deprotonation (by the first water). This would make it a BAC2 type. It turns out that if one makes the mechanism cyclic, the AAC2 and BAC2 annihilate each other in effect to create a single (peri)cyclic mechanism (which has no well known name, but might be referred to as the co-operative pathway). Such a mechanism can be extended using a third water molecule (magenta diagram); I will come to the reason for including that presently.
Why would one want to even consider such mechanisms? Because, if you look carefully, you will see no charges! Charge separation (= large dipole moment) takes energy. It is normally thought that this energy is more than compensated for by additional solvation (a process which is implicit rather than explicitly shown in text-book diagrams). But if you do not generate charge separation, you might not need that solvation energy. I will turn to quantum mechanics to try to decide what might be viable (I hesitate to use the term “going on”).
A ωB97XD/6-311G(d,p)/SCRF=water model (in which solvation is approximately included as a continuum model) calculation yields the following for the blue mechanism.
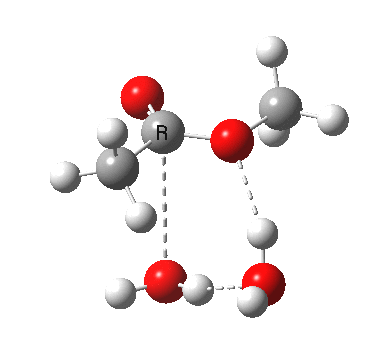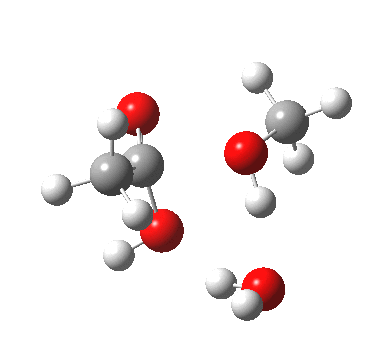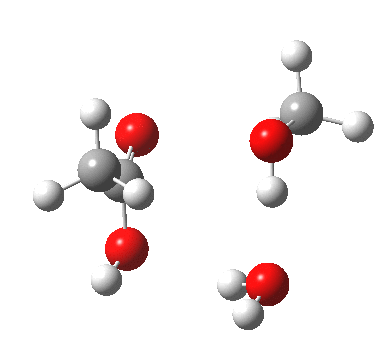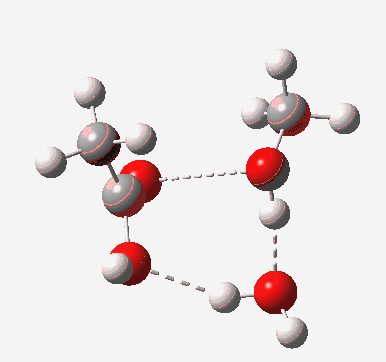 [cite]10.6084/m9.figshare.661351[/cite] [cite]10.6084/m9.figshare.661351[/cite] |
- Points to note are that it is concerted, in other words the quantum mechanics tells us that all the bonds CAN make and break in a single concerted process within a single kinetic step.
- The mechanism has an uncanny resemblance to the nucleophilic aromatic substitution I reported a couple of posts ago! It resembles an Sn2 displacement at an sp2 centre. Such juxtaposition of these two mechanisms is also not found in text-books. Recollect that with such aromatic substitution, it was possible to get both cncerted and stepwise mechanisms, depending on the substituents. Perhaps the same might be possible here?
- However, the energy barrier for the process with the substituents shown above (~45 kcal/mol) is rather too high (the experimental value is estimated as >22 kcal/mol[cite]10.1139/V09-011[/cite]). There may be at least three reasons for this;
- (a) a better solvation model would be needed to lower the energy,
- (b) the angles subtended at the transferring protons are strained (they optimally should be linear) and
- (c) water is a very poor general acid (or base)!
But as an answer in an examination, would the blue mechanism actually be wrong? You will have to ask the instructor setting the question how they might respond to that, although these authors[cite]10.1139/V09-011[/cite] certainly conclude that such a concerted mechanism is the more “correct”, at least for hydrolysis in water without added acid or base.
Point (b) above can be addressed by adding another water molecule, as per the magenta mechanism so as to enlarge the ring and reduce the angular strain. But before I present the results, I need to “normalise” the system by ALSO adding one (solvating) water molecule to the blue route, as below, so that we can directly compare the energies of the blue and magenta pathways.
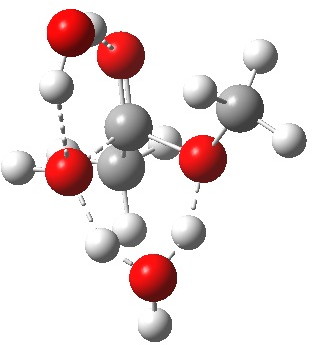
Click for 3D.
The result is a larger ring where the angular strain is clearly reduced. There is an entropic penalty for introducing that third water molecule, but despite this the free energy comes out 5.5 kcal/mol lower, and the activation barrier is also lower (~37 kcal/mol, still rather higher than experiment). It has been reported that incorporation of a 4th water molecule further improves matters[cite]10.1139/V09-011[/cite].
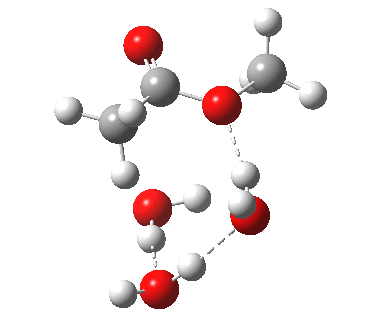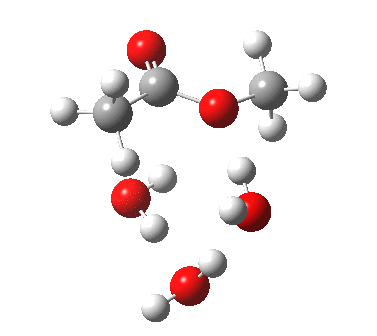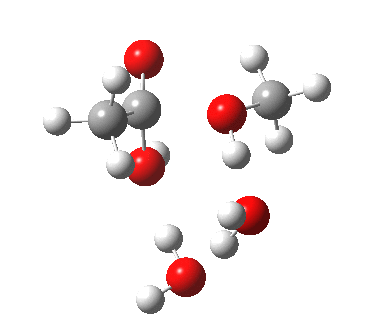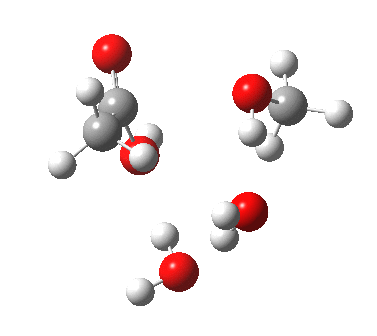 [cite]10.6084/m9.figshare.661789[/cite] [cite]10.6084/m9.figshare.661789[/cite] |
We can also address both points (b) and (c) above by replacing HA=H2O by HA=guanidiniumH+ (green), a better general acid. This polar modification introduces the ability for the system to better sustain charge separations, and indeed the initial product is now an ion pair tetrahedral intermediate (methoxide anion and guanidinium cation) carrying a dipole moment of 14.5D, an increase over the value for the transition state with three waters, 9.7D. The barrier (~21 kcal/mol) has gone in the opposite direction, decreasing significantly compared to the water catalysed reaction. The tetrahedral intermediate sits in an energy well of ~4 kcal/mol.
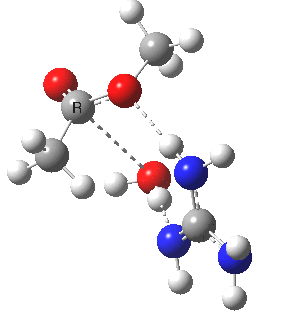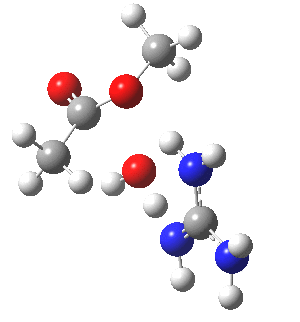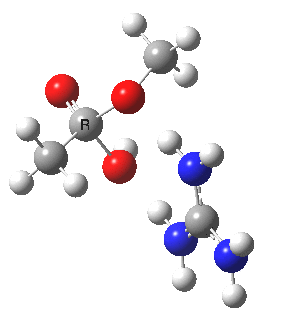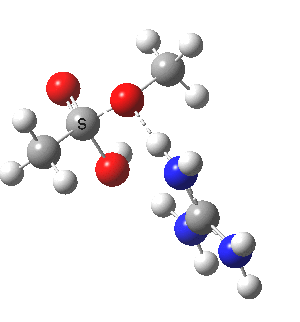 [cite]10.6084/m9.figshare.661791[/cite] [cite]10.6084/m9.figshare.661791[/cite] |
A second transition state exiting the tetrahedral intermediate has a free energy barrier[cite]10.6084/m9.figshare.661799[/cite] about 2.5 kcal/ol lower than the one entering it.
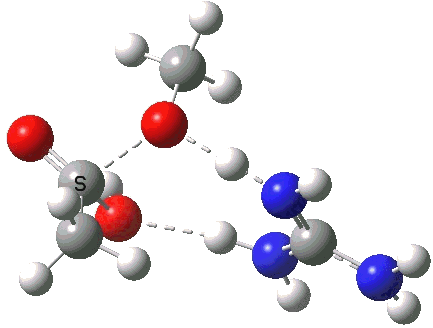
Click for 3D.
What might we have learnt? That ester hydrolysis using pure water could proceed through a cyclic and concerted transition state, involving three (or perhaps more) water molecules passing a proton baton along the chain, and in the process avoiding any large build up of charge separation. Replace two of these waters with say guanidine as a general acid/conjugate base capable of conjugatively stabilising charge-separated species and the mechanism changes to a stepwise reaction involving a dipolar tetrahedral intermediate sitting in a relatively shallow energy well.
Not possibly a picture that we might expect a student sitting an introductory examination in organic chemistry to reflect in its entirety, but also one that perhaps the text-books might start to hint at? Or: at some stage, armed merely with a “smart watch-cum-supercomputer”, a student taking such an exam might respond by performing the calculations described here as their submitted answer? Well, not for a year or two perhaps. But it has to be said that everything you see in this post was performed over less than two days of elapsed time, so these “reality checks” are not that time-consuming. Whether you choose to believe them or not of course is another matter.
Tags: ALSO, co-operative, energy, energy well, ester hydrolysis, free energy, Reaction Mechanism, shallow energy, solvation energy, Tutorial material