In the previous post, it was noted that Möbius annulenes are intrinsically chiral, and should therefore in principle be capable of resolution into enantiomers. The synthesis of such an annulene by Herges and co-workers was a racemic one; no attempt was reported at any resolution into such enantiomers. Here theory can help, since calculating the optical rotation [α]D is nowadays a relatively reliable process for rigid molecules. The rotation (in °) calculated for that Möbius annulene was relatively large compared to that normally measured for most small molecules.
Archive for April, 2009
The Chirality of Lemniscular Octaphyrins
Tuesday, April 28th, 2009The chirality of Möbius annulenes
Wednesday, April 22nd, 2009Much like climbing Mt. Everest because its there, some hypothetical molecules are just too tantalizing for chemists to resist attempting a synthesis. Thus in 1964, Edgar Heilbronner speculated on whether a conjugated annulene ring might be twistable into a Möbius strip. It was essentially a fun thing to try to do, rather than the effort being based on some anticipated (and useful) property it might have. If you read the original article (rumour has it the idea arose during a lunchtime conversation, and the manuscript was completed by the next day), you will notice one aspect of these molecules that is curious by its absence. There is no mention (10.1016/S0040-4039(01)89474-0) that such Möbius systems will be chiral. By their nature, they have only axes of symmetry, and no planes of symmetry, and such molecules therefore cannot be superimposed upon their mirror image; as is required of a chiral system (for a discussion of the origins and etymology of the term, see 10.1002/chir.20699).
A molecule with an identity crisis: Aromatic or anti-aromatic?
Monday, April 13th, 2009In 1988, Wilke[1] reported molecule 1
![gaytab A [24] annulene. Click on image for model.](http://www.ch.ic.ac.uk/rzepa/blog/wp-content/uploads/2009/04/gaytab.jpg)
A 24-annulene. Click for 3D.
It was a highly unexpected outcome of a nickel-catalyzed reaction and was described as a 24-annulene with an unusual 3D shape. Little attention has been paid to this molecule since its original report, but the focus has now returned! The reason is that a 24- annulene belongs formally to a class of molecule with 4n (n=6) π-electrons, and which makes it antiaromatic according to the (extended) Hückel rule. This is a select class of molecule, of which the first two members are cyclobutadiene and cyclo-octatetraene. The first of these is exceptionally reactive and unstable and is the archetypal anti-aromatic molecule. The second is not actually unstable, but it is reactive and conventional wisdom has it that it avoids the undesirable antiaromaticity by adopting a highly non-planar tub shape and hence instead adopts reactive non-aromaticity. Both these examples have localized double bonds, a great contrast with the molecule which sandwiches them, cyclo-hexatriene (i.e. benzene). The reason for the resurgent interest is that a number of crystalline, apparently stable, antiaromatic molecules have recently been discovered, and ostensibly, molecule 1 belongs to this select class!
References
- G. Wilke, "Contributions to Organo‐Nickel Chemistry", Angewandte Chemie International Edition in English, vol. 27, pp. 185-206, 1988. https://doi.org/10.1002/anie.198801851
How do molecules interact with each other?
Sunday, April 12th, 2009Understanding how molecules interact (bind) with each other when in close proximity is essential in all areas of chemistry. One specific example of this need is for the molecule shown below.
Aromatic electrophilic substitution: a different way of predicting regiospecificity
Saturday, April 4th, 2009
Every introductory course or text on aromatic electrophilic substitution contains an explanation along the lines of the resonance diagram shown below. With an o/p directing group such as NH2, it is argued that negative charge accumulates in those positions as a result of the resonance structures shown.
A lab in a backpack
Friday, April 3rd, 2009We recently developed a new computational chemistry practical laboratory here at Imperial College. I gave a talk about it at the recent ACS meeting in Salt Lake City. If you want to see the details of the lab, do go here. The talk itself contains further links and examples. Perhaps here I can quote only the final remark, namely that computational chemistry can now provide chemical accuracy for many problems, including spectroscopy and mechanism, and that the basic tools for doing it can easily be carried around in a backpack! Or, perhaps in the not to distant future, an iPhone!
On the importance of Digital repositories in Chemistry
Friday, April 3rd, 2009The preceeding blog entries contain stories about chemical behaviour. If you have clicked on the diagrams, you may even have gotten a Jmol view of the relevant molecules popping up. But if you are truly curious, you may even have the urge to acquire the relevant 3D information about the molecule, and play with it yourself. Even after 15 years of the (chemical) Web, this can be distressingly difficult to achieve (or can it be that it is only myself who wishes to view molecules in their native mode?). Thus the standard mechanism is to seek out on journal pages that disarming little entry entitled supporting information and to hope that you might find something useful embedded there. Embedded is the correct description, since the information is often found within the confines of an Acrobat file, and has to be extracted from there. Indeed, that is what I had to resort to in order to write one of the blog entries below. I ground my teeth whilst doing so.
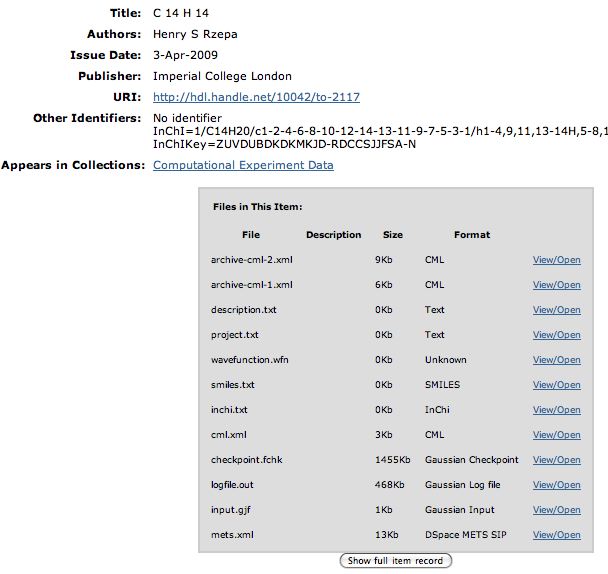
So is there a better way? We think so! The digital repository. If you click on this you should see the entry directly. What can you do there? Well, if you have suitable programs, you can download eg a Checkpoint file of the calculation that created the molecule model and re-activate it there. Or you can download just the CML file for viewing in any CML-compliant program (such as e.g. Jmol). Or you can check up on the InCHi string or the InChI Key of the molecule.
Pericyclic assistance for SN-1 solvolysis
Friday, April 3rd, 2009
Click on diagram to see model.