Archive for the ‘pericyclic’ Category
Monday, August 4th, 2014
The outcome of pericyclic reactions con depend most simply on three conditions, any two of which determine the third. Whether the catalyst is Δ or hν (heat or light), the topology determining any stereochemistry and the participating electron count (4n+2/4n). It is always neat to conjure up a simple switch to toggle these; heat or light is simple, but what are the options for toggling the electron count? Here is one I have contrived by playing a game with the periodic table.  The ring closure of a divinylketone is called the Nazarov reaction, it being promoted thermodynamically by coordination of a Lewis acid to atom X. Divinyl ketone can be regarded as a hidden pentadienyl cation, since the C=O bond is polarised Cδ+Oδ- in the time-honoured manner of organic chemistry. In this (formal) resonance form, it becomes part of a pentadienyl cation and can electrocyclise via a 4-electron reaction involving a stereochemical process known as conrotation. The new bond is formed antarafacially (from opposite faces) at the termini of the pentadienyl cation (ωB97XD/6-311G(d,p)/SCRF=dichloromethane.[1]). Note that for the uncatalysed reaction, the barrier is high and the reaction is endothermic but adding a BF3 to the oxygen lowers the barrier and removes the endothermicity.[2]
The ring closure of a divinylketone is called the Nazarov reaction, it being promoted thermodynamically by coordination of a Lewis acid to atom X. Divinyl ketone can be regarded as a hidden pentadienyl cation, since the C=O bond is polarised Cδ+Oδ- in the time-honoured manner of organic chemistry. In this (formal) resonance form, it becomes part of a pentadienyl cation and can electrocyclise via a 4-electron reaction involving a stereochemical process known as conrotation. The new bond is formed antarafacially (from opposite faces) at the termini of the pentadienyl cation (ωB97XD/6-311G(d,p)/SCRF=dichloromethane.[1]). Note that for the uncatalysed reaction, the barrier is high and the reaction is endothermic but adding a BF3 to the oxygen lowers the barrier and removes the endothermicity.[2] 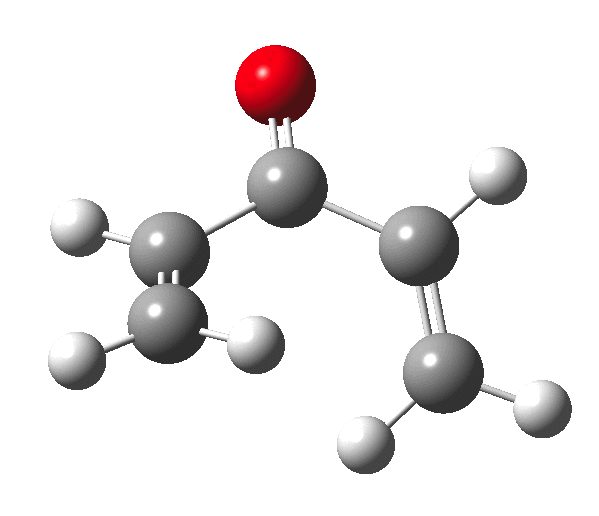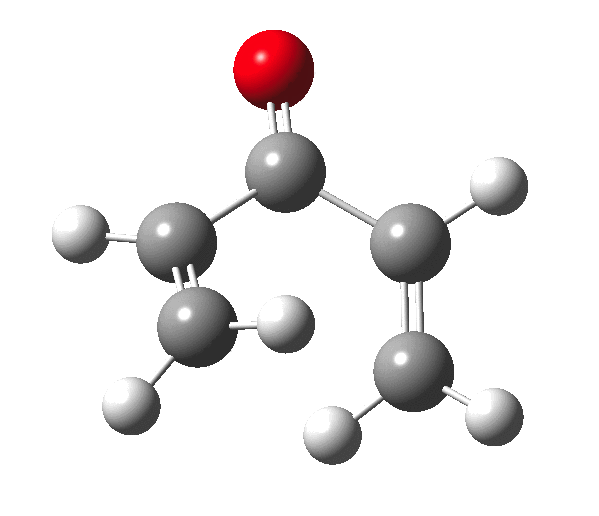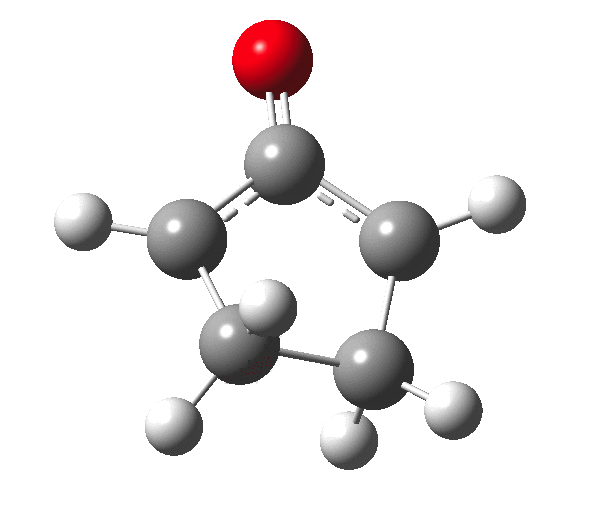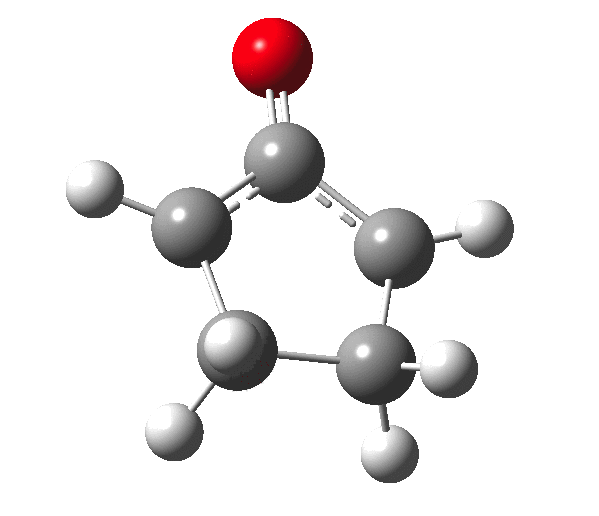

 So, one can play a game and ask what would happen if the polarity of the C=X bond were to be reversed. This means going left of oxygen in the periodic table, ending at Be.[3] The reaction has a high barrier, but it is strongly exothermic.† However the most noteworthy aspect is that the stereochemistry of the electrocyclisation is now disrotatory, with suprafacial bond formation (from the bottom face in the animation below). The stereochemical outcome of this reaction has been flipped by reversing the polarity of the CX bond.‡
So, one can play a game and ask what would happen if the polarity of the C=X bond were to be reversed. This means going left of oxygen in the periodic table, ending at Be.[3] The reaction has a high barrier, but it is strongly exothermic.† However the most noteworthy aspect is that the stereochemistry of the electrocyclisation is now disrotatory, with suprafacial bond formation (from the bottom face in the animation below). The stereochemical outcome of this reaction has been flipped by reversing the polarity of the CX bond.‡ 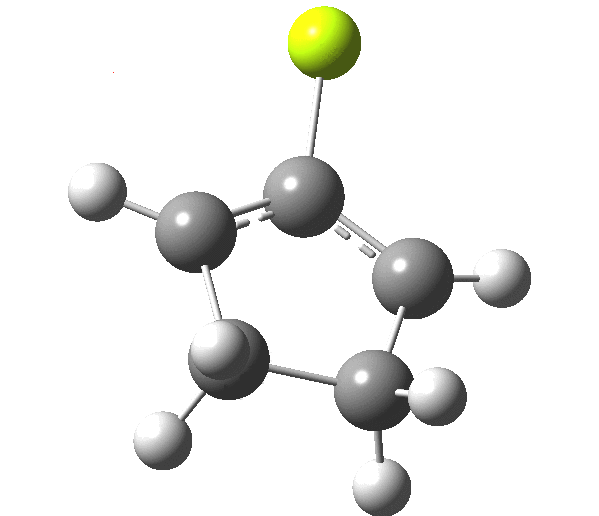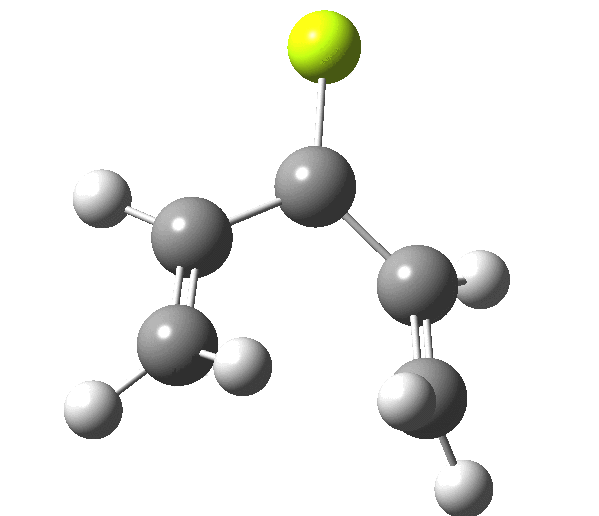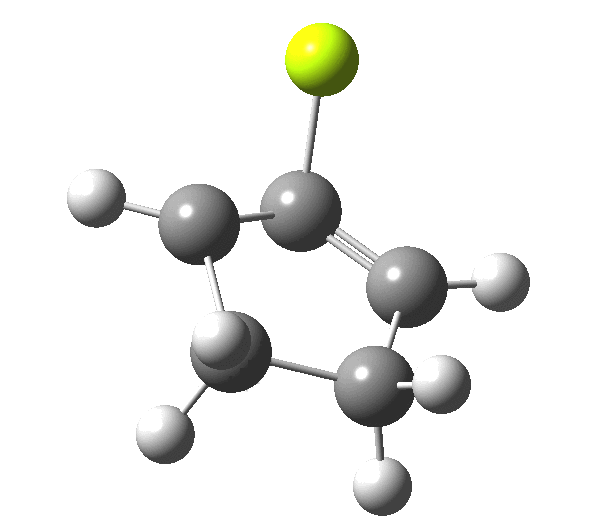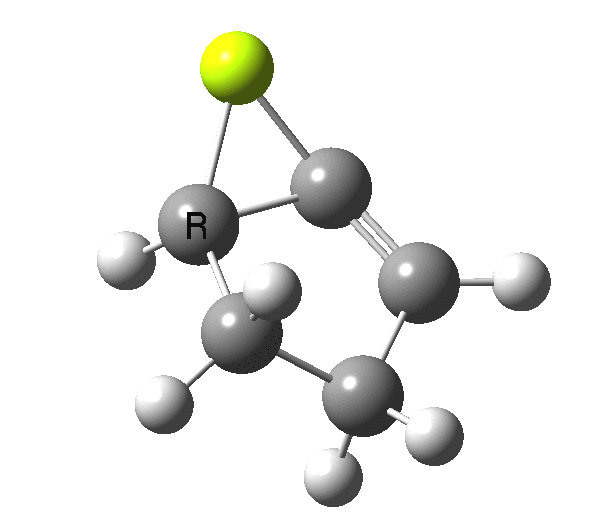
 This little example shows how a thought game played using the periodic table can then be reality tested by solving appropriate quantum mechanical equations. In this instance, one is not going to rush into the laboratory to try to replicate the experiment, but it might help catalyse new thoughts amongst the readers of this blog.
This little example shows how a thought game played using the periodic table can then be reality tested by solving appropriate quantum mechanical equations. In this instance, one is not going to rush into the laboratory to try to replicate the experiment, but it might help catalyse new thoughts amongst the readers of this blog.
(more…)
References
- H.S. Rzepa, "Gaussian Job Archive for C5H6O", 2014. https://doi.org/10.6084/m9.figshare.1125721
- H.S. Rzepa, "Gaussian Job Archive for C5H6BF3O", 2014. https://doi.org/10.6084/m9.figshare.1125724
- H.S. Rzepa, "Gaussian Job Archive for C5H6Be", 2014. https://doi.org/10.6084/m9.figshare.1125792
Tags:animation
Posted in Interesting chemistry, pericyclic | No Comments »
Sunday, February 16th, 2014
The potential energy surface for a molecule tells us about how it might react. These surfaces have been charted for thousands of reactions using quantum mechanics, and their basic features are thought to be well understood. Coming across an entirely new feature is rare. So what do you make of the following?
(more…)
Tags:potential energy surface
Posted in pericyclic, reaction mechanism | 11 Comments »
Thursday, January 2nd, 2014
As my previous post hints, I am performing my annual spring-clean of lecture notes on pericyclic reactions. Such reactions, and their stereochemistry, are described by a set of selection rules. I am always on the lookout for a simple example which can most concisely summarise these rules. The (hypothetical) one shown below I think nicely achieves this, and raises some interesting issues in the process.
(more…)
Posted in pericyclic, reaction mechanism | No Comments »
Sunday, December 29th, 2013
When I first started giving lectures to students, it was the students themselves that acted as human photocopiers, faithfully trying to duplicate what I was embossing on the lecture theatre blackboard with chalk. How times have changed! Here I thought I might summarise my latest efforts to refactor the material I deliver in one lecture course on pericyclic reactions (and because my notes have always been open, you can view them yourself if you wish).
(more…)
Tags:chemical diagram, chemical drawing software, Internet Explorer, Microsoft, Microsoft Windows
Posted in General, pericyclic | 2 Comments »
Wednesday, November 13th, 2013
Not long ago, I described a cyclic carbene in which elevating the carbene lone pair into a π-system transformed it from a formally 4n-antiaromatic π-cycle into a 4n+2 aromatic π-cycle. From an entirely different area of chemistry, another example of this behaviour emerges; Schreiner’s[1] trapping and reactions of t-butyl-hydroxycarbene, as described on Steve Bachrach’s blog. A point I often make is that chemistry is all about connections, and so here I will discuss such a connection.
(more…)
References
- D. Ley, D. Gerbig, and P.R. Schreiner, "Tunneling control of chemical reactions: C–H insertion versus H-tunneling in tert-butylhydroxycarbene", Chem. Sci., vol. 4, pp. 677-684, 2013. https://doi.org/10.1039/c2sc21555a
Tags:Steve Bachrach
Posted in pericyclic, reaction mechanism | No Comments »
Wednesday, June 26th, 2013
A reader asked me about the mechanism of the reaction of 2-picoline N-oxide with acetic anhydride to give 2-acetoxymethylpyridine (the Boekelheide Rearrangement[1]). He wrote ” I don’t understand why the system should prefer to go via fragmentation-recombination (… the evidence being that oxygen labelling shows scrambling) when there is an easy concerted pathway available (… a [3,3]sigmatropic shift). Furthermore, is it possible for two pathways to co-exist?” Here is how computation might enlighten us.
(more…)
References
- A. Massaro, A. Mordini, A. Mingardi, J. Klein, and D. Andreotti, "A New Sequential Intramolecular Cyclization Based on the Boekelheide Rearrangement", European Journal of Organic Chemistry, vol. 2011, pp. 271-279, 2010. https://doi.org/10.1002/ejoc.201000936
Tags:ATM, CF 3 CO, CH 3 CO, extraneous product, free energy, free energy barrier, recombination
Posted in Interesting chemistry, pericyclic, reaction mechanism | 3 Comments »
Monday, May 20th, 2013
Sometimes the originators of seminal theories in chemistry write a personal and anecdotal account of their work. Niels Bohr[1] was one such and four decades later Robert Woodward wrote “The conservation of orbital symmetry” (Chem. Soc. Special Publications (Aromaticity), 1967, 21, 217-249; it is not online and so no doi can be given). Much interesting chemistry is described there, but (like Bohr in his article), Woodward lists no citations at the end, merely giving attributions by name. Thus the following chemistry (p 236 of this article) is attributed to a Professor Fonken, and goes as follows (excluding the structure in red):
(more…)
References
- N. Bohr, "Der Bau der Atome und die physikalischen und chemischen Eigenschaften der Elemente", Zeitschrift f�r Physik, vol. 9, pp. 1-67, 1922. https://doi.org/10.1007/bf01326955
Tags:electrocyclic, energy, final product, free energy, Gerhard Fonken, Historical, Niels Bohr, pericyclic, professor, Reaction Mechanism, Robert Woodward, Woodward
Posted in pericyclic | 4 Comments »
Thursday, December 20th, 2012
I have written earlier about dihydrocostunolide, and how in 1963 Corey missed spotting the electronic origins of a key step in its synthesis.[1]. A nice juxtaposition to this failed opportunity relates to Woodward’s project at around the same time to synthesize vitamin B12. The step in the synthesis that caused him to ponder is shown below.
(more…)
References
- E.J. Corey, and A.G. Hortmann, "The Total Synthesis of Dihydrocostunolide", Journal of the American Chemical Society, vol. 87, pp. 5736-5742, 1965. https://doi.org/10.1021/ja00952a037
Tags:dispersion energy, free energy, Historical, pericyclic
Posted in Interesting chemistry, pericyclic, reaction mechanism | No Comments »
Thursday, April 2nd, 2009
Mauksch and Tsogoeva have recently published an article illustrating how a thermal electrocyclic reaction can proceed with distoratory ring closure, whilst simultaneously also exhibiting 4n electron Möbius-aromatic character[1]. Why is this remarkable? Because the simple Woodward-Hoffmann rules state that a disrotatory thermal electrocyclic reaction should proceed via a Hückel-aromatic 4n+2 electron transition state. Famously, Woodward and Hoffmann stated there were no exceptions to this rule. Yet here we apparently have one! So what is the more fundamental? The disrotatory character, or the 4n/Möbius character in the example above? Mauksch and Tsogoeva are in no doubt; it is the former that gives, and the latter is correct.
(more…)
References
- M. Mauksch, and S. Tsogoeva, "A Preferred Disrotatory 4<i>n</i> Electron Möbius Aromatic Transition State for a Thermal Electrocyclic Reaction", Angewandte Chemie International Edition, vol. 48, pp. 2959-2963, 2009. https://doi.org/10.1002/anie.200806009
Tags:Hoffmann, Interesting chemistry, jmol, NICS, pericyclic, Reaction Mechanism, Steve Bachrach
Posted in pericyclic | 1 Comment »
The ring closure of a divinylketone is called the Nazarov reaction, it being promoted thermodynamically by coordination of a Lewis acid to atom X. Divinyl ketone can be regarded as a hidden pentadienyl cation, since the C=O bond is polarised Cδ+Oδ- in the time-honoured manner of organic chemistry. In this (formal) resonance form, it becomes part of a pentadienyl cation and can electrocyclise via a 4-electron reaction involving a stereochemical process known as conrotation. The new bond is formed antarafacially (from opposite faces) at the termini of the pentadienyl cation (ωB97XD/6-311G(d,p)/SCRF=dichloromethane.[1]). Note that for the uncatalysed reaction, the barrier is high and the reaction is endothermic but adding a BF3 to the oxygen lowers the barrier and removes the endothermicity.[2]

So, one can play a game and ask what would happen if the polarity of the C=X bond were to be reversed. This means going left of oxygen in the periodic table, ending at Be.[3] The reaction has a high barrier, but it is strongly exothermic.† However the most noteworthy aspect is that the stereochemistry of the electrocyclisation is now disrotatory, with suprafacial bond formation (from the bottom face in the animation below). The stereochemical outcome of this reaction has been flipped by reversing the polarity of the CX bond.‡

This little example shows how a thought game played using the periodic table can then be reality tested by solving appropriate quantum mechanical equations. In this instance, one is not going to rush into the laboratory to try to replicate the experiment, but it might help catalyse new thoughts amongst the readers of this blog.