An extensive discussion developed regarding my post on a fascinating helical [144]-annulene. Topics included the nature of the ring current sustained by the π-electrons and in particular the bond-length alternation around the periphery and whether this should alter if the electron count were to be changed to that of a 4n+2 system (i.e. a dication). Whilst the [144]-annulene itself is hypothetical, it emerged that some compounds known as expanded porphyrins have very similar (albeit smaller scale) helical structures. X-ray structures for two such provide useful reality checks on the calculations. Here‡ I include the (3D) coordinates of these two systems so that you can explore for yourself their helicity.
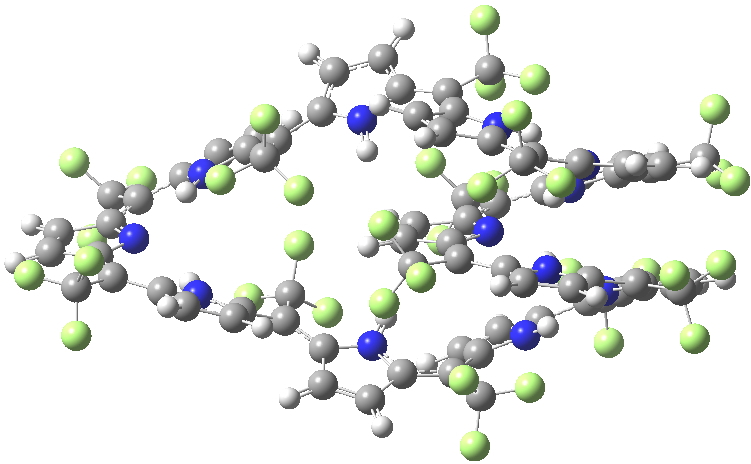
SELQUW. Click for 3D X-ray structure
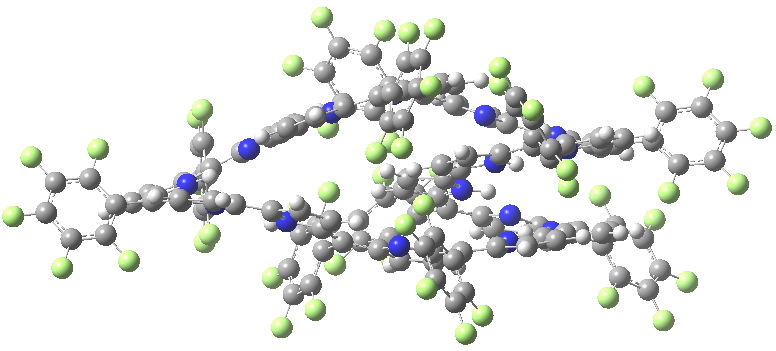
HIYTAL. Click for 3D X-ray structure
I include below Δrmeso, being the mean unsigned difference in bond length (Å) at the meso positions of the porphrin ring, the calculations being at the 6-311G(d,p) level using the DFT procedure indicated below. The linking number analysis[1] for such systems will be reported elsewhere.[2]
| Method | SELQUW | HIYTAL |
| X-ray | 0.048 | 0.045 |
| B97D/6-311G(d,p) | 0.025 | 0.015 |
| B3LYP/6-311G(d,p) | 0.047 | 0.017 |
‡The WordPress system operated here does not enable 3D coordinates to be inserted into the comment section of a post, only the main body.
References
- S.M. Rappaport, and H.S. Rzepa, "Intrinsically Chiral Aromaticity. Rules Incorporating Linking Number, Twist, and Writhe for Higher-Twist Möbius Annulenes", Journal of the American Chemical Society, vol. 130, pp. 7613-7619, 2008. https://doi.org/10.1021/ja710438j
- H.S. Rzepa, "Lemniscular Hexaphyrins as Examples of Aromatic and Antiaromatic Double-Twist Möbius Molecules", Organic Letters, vol. 10, pp. 949-952, 2008. https://doi.org/10.1021/ol703129z
Tags: Helical annulenes, X-ray