George Olah passed away on March 8th. He was part of the generation of scientists in the post-war 1950s who had access to chemical instrumentation that truly revolutionised chemistry. In particular he showed how the then newly available NMR spectroscopy illuminated structures of cations in solvents such “Magic acid“. The obituaries will probably mention his famous “feud” with H. C. Brown over the structure of the norbornyl cation (X=CH2+), implicated in the mechanism of many a solvolysis reaction that characterised the golden period of physical organic chemistry just before and after WWII.
The dispute between Olah and Brown was not played on a pitch using quite the same goal posts. Olah did much of his work in magic acid and Brown did his in aqueous solutions. I was involved in a tiny way when the discussion about the precise character of the norbornyl cation was reaching its peak in the mid 1970s. At the time, I was working with Michael Dewar, who was himself not shy in joining in the fun and sometimes very acrimonious disputes at conferences. We contributed by calculating the so-called core-electron carbon ESCA spectrum.[1] History records that we came down on the wrong side,‡ by suggesting that this form of spectroscopy supported Brown rather than Winstein/Olah on the basis of a 6:1 spectral deconvolution (classical) rather than 5:2 (non-classical). More recently of course the crystal structure of the parent cation itself has been shown to be non-classical[2] (there are other crystal structures which differ in respect to having one or more additional methyl groups[3]). For a 3D model of norbornyl cation, see DOI: 10.5517/CCZ21LN. This still leaves the issue (very slightly) open for the structure of the solvated cation when formed in water!
When I started to teach a course in molecular modelling, I touched briefly on how modelling could contribute and whilst updating the notes in the 1990s, wondered why the boron analogue had never been so studied (X=BH2). Unlike the crystallographically difficult norbornyl ion-pair, the iso-electronic boron species would be neutral and not need a counter-ion. Perhaps it might be a more manageable molecule? Checking the Cambridge structural database, such a species has never been reported!† So here as my homage to Olah, I report its calculated structure (b2plypd3/Def2-TZVPP, DOI: 10.14469/hpc/2236).
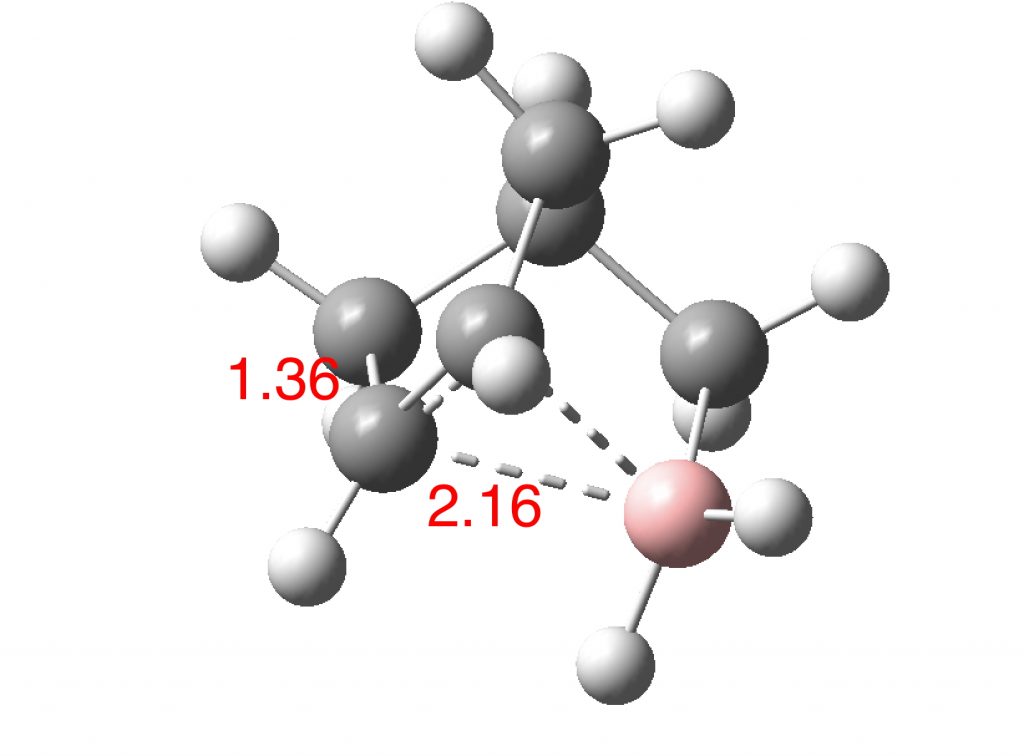
The norbornyl cation has symmetrical C-C bridging distances of ~1.80±0.02Å and a basal C-C distance of ~1.39±0.02Å. The calculated values for the boron equivalent are 2.16Å and 1.36Å respectively, with all positive force constants. B-C bonds are normally 1.66-1.72Å, significantly longer than C-C bonds, which makes the longer B-C lengths in this example unsurprising. More interestingly, the species has one vibrational normal mode (ν 203 cm-1) which corresponds to the [1,2] shift of the BH2 group across the basal C-C. For a classical species, this vibrational motion would correspond to a transition state (an imaginary vibration) but for a non-classical species it is of course real. In this sense it is analogous to the so-called real Kekulé mode in non-classical benzene, which “equilibrates” the two classical Kekulé structures. The corresponding calculated vibration for the norbornyl cation itself is ν 194 cm-1 (DOI: 10.14469/hpc/2238).
Of course, the entire controversy over the structure of this species is littered with comparisons between not quite similar systems, differing in a methyl group more or less. So morphing a C+ to a B might be seen as quite a large change. But perhaps if it had been crystallised in say the 1960s, would the subsequent debates have taken a different turn?
‡ We were also wrong about the symmetry of the Diels-Alder cyclisation, which is nowadays accepted to be synchronous rather than asynchronous for simple Diels-Alder reactions. But that is another story.
†GAXLIA is perhaps the closest analogue.[4],
References
- M.J.S. Dewar, R.C. Haddon, A. Komornicki, and H. Rzepa, "Ground states of molecules. 34. MINDO/3 calculations for nonclassical ions", Journal of the American Chemical Society, vol. 99, pp. 377-385, 1977. https://doi.org/10.1021/ja00444a012
- https://doi.org/
- T. Laube, "Redetermination of the Crystal Structure of the 1,2,4,7‐<i>anti</i>‐tetramethylbicyclo[2.2.1]heptan‐2‐yl cation at 110 K", Helvetica Chimica Acta, vol. 77, pp. 943-956, 1994. https://doi.org/10.1002/hlca.19940770407
- P.J. Fagan, E.G. Burns, and J.C. Calabrese, "Synthesis of boroles and their use in low-temperature Diels-Alder reactions with unactivated alkenes", Journal of the American Chemical Society, vol. 110, pp. 2979-2981, 1988. https://doi.org/10.1021/ja00217a053
Tags: 2-Norbornyl cation, aqueous solutions, Chemical bond, chemical instrumentation, Chemistry, George Andrew Olah, George Olah, Ion association, Magic acid, Michael Dewar, Molecule, Nature, Physical organic chemistry, Reactive intermediates, spectroscopy