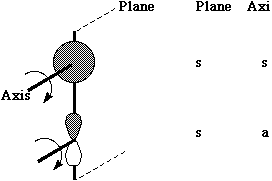

One can take this one step further by considering the symmetry properties of molecular orbitals formed by the overlap of two or more atomic orbitals;
MOs formed from two overlapping sigma orbitals:
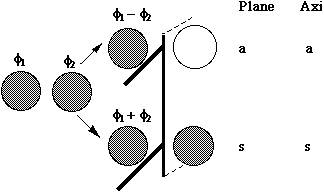
MOs formed from two overlapping p orbitals (sigma bonds):
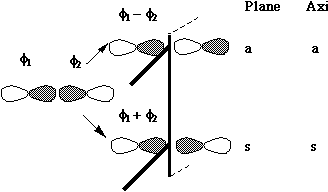
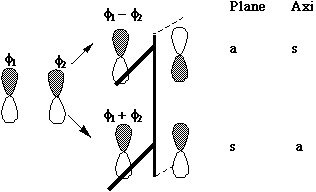
MOs formed from two parallel overlapping p orbitals (p bonds):
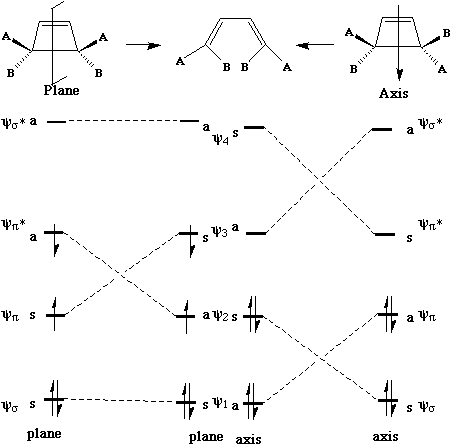
We can now use these basic orbitals to construct the relevant molecular orbitals for two interconverting molecules, cyclobutene and butadiene, with the purpose of following how these two sets of orbitals change when one molecule is converted into the other. Note particularly that we need only construct the MOs explicitly involved in the reaction; most of the sigma framework remains unchanged and no orbitals derived from this need to be considered:
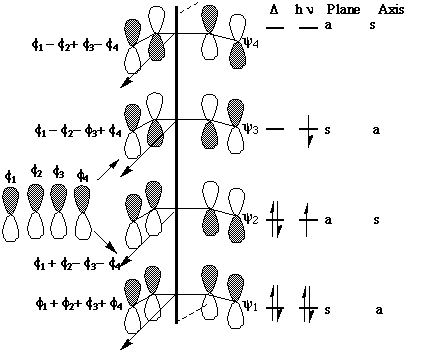
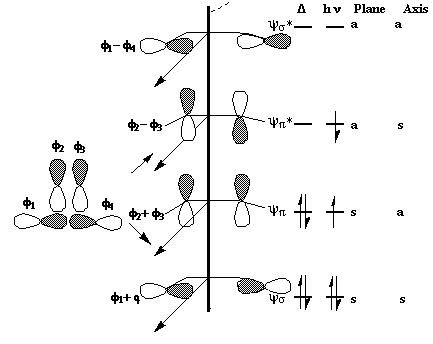
In order to interconvert cyclobutene and butadiene, the four MOs labelled Y1, Y2, Y3, Y4 must be
converted into Ys, Yp, Yp*, Ys*. There are two stereochemically distinct ways in which this might
be accomplished;
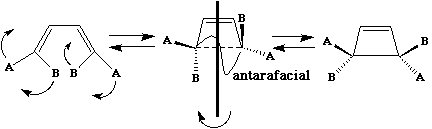
conrotation;
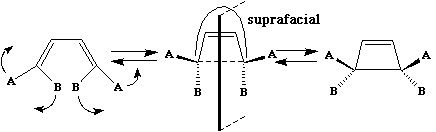
disrotation;
This enables a correlation diagram for the reaction to be constructed, according to the following
rules: no two orbitals of the same symmetry can cross during the reaction, whilst orbitals of different
symmetry can cross. The favoured pathway is the one which results in a product of the same
electronic excitation as the reactant. A pathway which results in the product in a higher electronic
state than the reactant is said to be "forbidden".
Whilst this rule is normally followed fairly well for ground states, it can be overturned when for
example steric or geometrical strain in the "allowed" pathway promotes the "forbidden" route. The
situation is actually more complex for photochemical reactions, and much recent evidence suggests
that the Woodward-Hoffmann rules are not always followed see note 1.
These correlation diagrams can be generalised for any electrocyclic reaction with appropriate
symmetry. However, correlation diagrams are less readily applied for reactions with no symmetry.
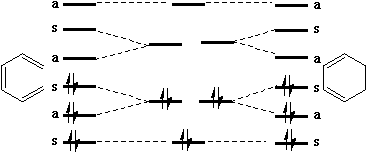
Dewar and Zimmerman independently noticed that the 'topological' properties of these correlation
diagrams are very similar to those obtained using eg Huckel theory for aromatic molecules. For
example, the diagram for the electrocyclic conversion of hexatriene to cyclohexadiene is remarkably
similar at the transition state to the ground state orbitals of benzene;
Huckel was also able to show that if a cyclic conjugated p system is irradiated with light so that it goes
into the first 'excited' electronic state, it is especially stable if the number of cyclically conjugated
electrons equals 4n Hence photochemically activated pericyclic reactions will proceed suprafacially
via a Huckel transition state if the electron count corresponds to 4n. The antarafacial mode
described above has no counterpart in a stable aromatic molecule, as benzene did for the suprafacial
mode. An antarafacial mode could be formed by taking benzene and giving the p system a 180š
twist. If this is done, the resultant 'twisted' benzene has been termed 'Mobius Benzene'.
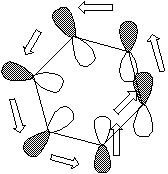
Here the electron density continuity passes through the plane of the molecule, from the top face through to the bottom face. Such a MOBIUS system it turns out is especially stable if it contains 4n electrons (or 4n+2 if photochemically excited). These four conditions are most easily summarised as follows;
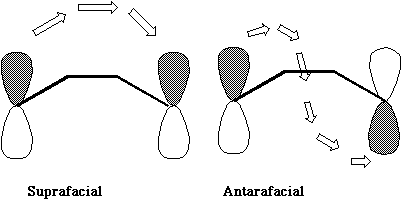
The antarafacial mode involves a 'twisting' of the orbitals so that the two components of the new s bond come from OPPOSITE faces of the reactant p system. If two or more sigma bonds form during the reaction, as in cycloaddition reactions, then the overlap of the HOMO of one reactant with the LUMO of the second reactant must be considered. For simple systems, the form of the HOMO and LUMO is not difficult to remember. For more complex systems, explicit calculations have to be carried out and the Frontier Orbital method becomes more difficult to apply. The advantage of the "FMO" method is that it can be expressed quantitatively in terms of the magnitude of the coefficients involved, and can hence be used to predict regioselectivity etc.