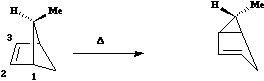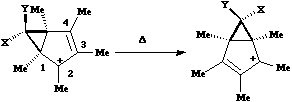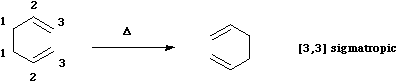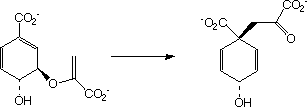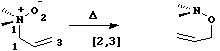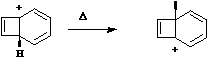7. Examples Of Sigmatropic Reactions.
There are two different types of sigmatropic reaction, a) those that involve the migration of a
hydrogen atom and b) those that involve a carbon or other atom. In the former category, the
hydrogen atom can migrate either suprafacially or antarafacially across the conjugated system,
leading to Huckel or Möbius topology for the transition states;
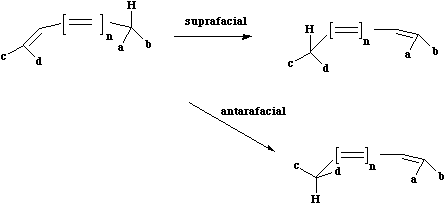
Something different can occur when a carbon migrates.
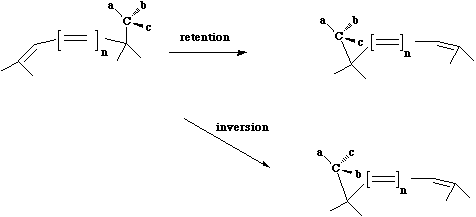
The configuration at the migrating centre can
be either retained (= suprafacial mode = Huckel topology) or inverted (= antarafacial mode = Möbius
topology). Note that Möbius transition states are relatively common in this class because there is
often little strain involved in inversion of configuration at a carbon.
The most common category of hydrogen shift involves a so called [1,5] sigmatropic shift. As seen
below this involves 6 electrons, and hence falls into the 4n+2 thermal category.
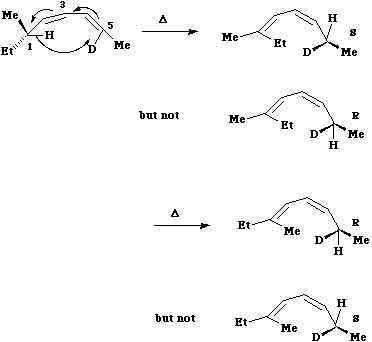
Here Huckel
topology induces a suprafacial Hydrogen shift. The example shown involves a 'transfer of chirality'
from the left hand side of the molecule to the right hand side. The absolute configuration of the two
formed products shows unambiguously that the transfer of the hydrogen atom must occur
suprafacially along the alkene.
Another illustration of [1,5] hydrogen shifting can be seen in cyclopentadiene. The structure of this
species implies that the 1H nmr spectrum should contain a resonance at about 2 ppm corresponding to
the sp3 protons and a second peak at about d 5 corresponding to the sp2 protons, the ratio being 1:2.
In fact, the room temperature spectrum shows only one peak at about 4 ppm. The reason for this is that
a series of successive [1,5] hydrogen shifts is occurring so quickly that only the 'averaged' proton
position is manifested in the nmr spectrum.
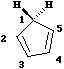
Slowing down the exchange by cooling produces the
expected spectrum described above! Many nmr spectra are affected by these so called 'degenerate'
pericyclic processes, also called "ring whizzing".
The next example illustrates a carbon [1,3] migration.
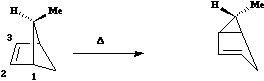
This involves 4 electrons, is hence a 4n
process requiring Möbius topology, and this can be most easily achieved by inverting the
configuration of the migrating carbon atom. If a model of this reaction is constructed, one can see
that the configuration of the migrating sp3 carbon is indeed inverted.
Next a cationic species, and from the numbering, it is classified as a [1,4]
carbon migration.
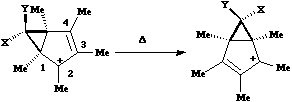
Again, the electron count corresponds to 4n, and again inversion at the migrating
carbon atom is required. In this example this inversion is actually directly illustrated by inspecting
the nmr spectrum. For the case where X and Y = Me, three and only three distinct methyl peaks are
observed in the 1H nmr spectrum. This would only occur if [1,4] migration is fast, and also if
inversion at the migrating centre were occurring, because this retains the individual identity of the
two methyl groups X and Y. If retention at the migrating centre were to occur, X and Y would
interchange their identity, and only two methyl peaks would have been observed.
Note that if two more electrons were to be added to this system (ie the carbanion), the nmr spectrum
would indeed be predicted to have only two methyl resonances.
As the cyclopropyl group "whizzes" around the ring, inversion at the carbon occurs, ensuring the CN
group is always endo with respect to the large ring.
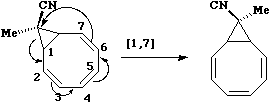
The next example is the one given in the
introduction; the "Cope" rearrangement;
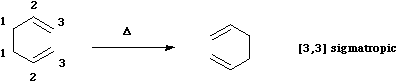
Here, an allyl group is migrating rather than a single carbon atom. The electron count is 6 (4n+2) and
the reaction is thermally allowed via Huckel topology with suprafacial components. When an oxygen atom
is involved, the reaction is called a "Claisen rearrangement". A very rare (indeed unique!)
example of an enzymically
catalysed sigmatropic reaction involves the Claisen type conversion of
"chorismate" to "prephenate"
by Chorismate mutase;
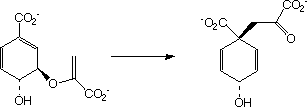
Much elegant work in this area was done by the Paul Bartlett group. Read all about them here.
When heteroatoms are involved, sigmatropic rearrangements involve odd numbers of atoms;
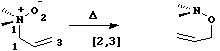
Finally in this category, another conundrum. Can you classify the following reaction, and decide
if it is thermally allowed or not?
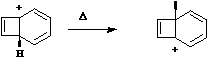
Return to index