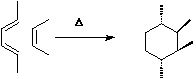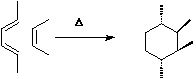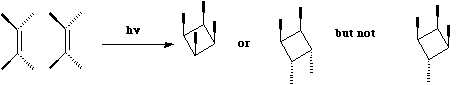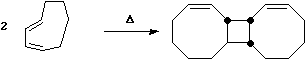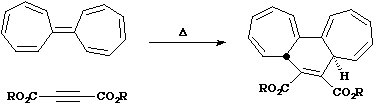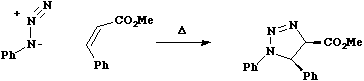6. Examples Of Cycloaddition/Elimination Reactions.
The simplest example is the well known Diels Alder cycloaddition. Twop systems are involved, one
contributing 4p electrons, the other 2p electrons. The total count is 6 (4n+2, n=1) and since the
reaction is thermal, it must proceed via Huckel topology involving suprafacial components. These
are considered as follows. Eachp systems forms two new s bonds. These two new bonds can either
form on the SAME face of onep system (suprafacial) or from opposite faces of onep system
(antarafacial). A system of nomenclature has been developed to describe this. Hence the system
below is classified as a p4s + p2s cycloaddition.
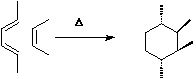
The only known example of an enzymically catalysed Diels-Alder reaction
is the unique enzyme extracted from Alternaria Solani which catalyses
a [2+4} cycloaddition in prosolanapyrone (III) with very high exo-selectivity (the normal
selectivity is endo).
For more details, see J. Chem. Soc., Chem. Commun, 1995, 1321.
The Frontier orbital approach is getting more complicated, and this is the last example we will
consider in detail. The HOMO of one molecule and the LUMO of the other must overlap in phase. In
this example, there are therefore two combinations that must be considered; the HOMO of the
"diene" with the LUMO of the "ene", and the LUMO of "diene" with the HOMO of "ene". For this
example, both give the same result, but this need not always be the case.
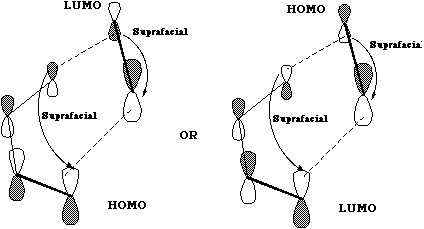
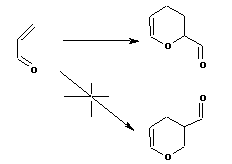
An extension of this frontier orbital approach is to state that the two LARGEST orbital nodes will
overlap with one another, and the two SMALLEST will also match up. The size of the orbitals must
be obtained from detailed quantum mechanical calculations. Such considerations can explain much
pericyclic regiochemistry, as for example in the dimerisation of acrolein;
One final extension of this approach, to consider not just the primary HOMO/LUMO overlap but
other overlaps as well (so called secondary orbital overlaps) is required to explain why the Diels
Alder reaction shows an endo rather than an exo preference. This however is beyond the scope of
the present lectures.
This is a 4 electron (4n) reaction,
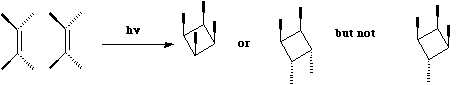
and photochemically proceeds via a Huckel topology (p2s + p2s).
Thermally, the dimerisation of eg ethene is predicted by the selection rules to proceed as a p2s +
p2a, the subscript a indicating an antarafacial component must be present on one alkene. However,
there is a problem; an antarafacial mode implies a Mobius like twisting of 180¡ in the p system of
the ethene, but ethene has only two carbons and cannot be twisted so much! Quantitative models
indicate that only about 130¡ of twisting can actually be induced, of which about 80¡ occurs on one
ethene and 50¡ occurs by twisting one ethene with respect to the other. Strain is also relieved by
forming the two bonds at different rates.
The trans alkene shown below has sufficient driving force to actually indulge in this rather twisted
transition state. Note that only the two strained trans double bonds react; the more stable cis double
bonds do not participate in the reaction.
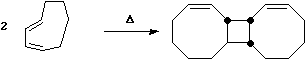
A more common example of a presumed p2s + p2a reaction is the reaction between a ketene (or
keteneimine) and alkene;
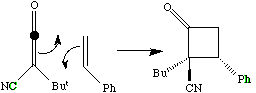
The antarafacial component is present only in the transition state, and it has the effect of giving the
more sterically hindered product!It was noted above that one way of relieving strain induced by an
antarafacial component is to make the two forming bonds of unequal length. In the extreme, for the
ketene example above, this corresponds to;
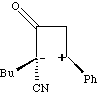
This of course is a stepwise rather than concerted reaction and is no longer a pericyclic process.
Many 2+2 cycloadditions are thought to proceed in this manner, especially in more polar solvents
which stabilise the intermediate zwitterions. In other systems, such asymmetry corresponds to the
formation of so called "biradical intermediates", and recent evidence suggest these routes may be
more common than hitherto suspected!
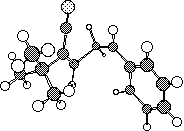
In the next example, the reaction is classified as a p2s + p14a reaction, since 16 electrons = 4n
(n=4) and hence again requires one antarafacial component.
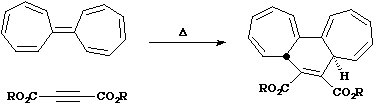
One point to note is that the antarafacial
component could in principal occur on either alkene, but in practice the geometrical distortion is
such that either the more strained or the larger component bears the antarafacial mode.
As before, lone pairs count as two electrons, making the next example a six electron system; p2s +
p4s
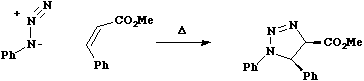
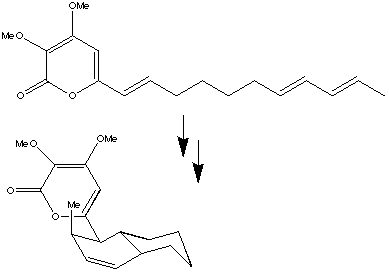
The formation of endrianic acid involves the creation of eight chiral centres from a simple linear
polyene precursors. This consists in sequence of pericyclic reactions to give the four membered ring system.
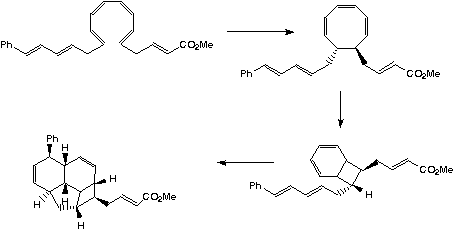
A subsequent intramolecular p2s +
p4s Diels Alder cycloaddition gives the final product.
Return to index