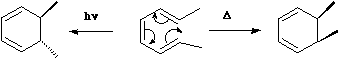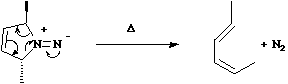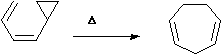3. Principal Categories of Organic Pericyclic Reactions.
Here we make no attempt to describe the
rich variety of organometallic and inorganic reactivity that can also be described as pericyclic. There
are four major organic categories, of which we will discuss three in rather greater detail than the
fourth.
Electrocyclic Reactions.
An electrocyclic reaction involves the concerted formation of a sigma bond
between the two ends of a linear conjugated ¼ system, or the reverse reaction in which the sigma bond is
broken to produce a linear conjugated system.
In these definitions, by 'conjugated', we mean a
system of C-C multiple bonds, or of lone pairs. Exceptions Also;
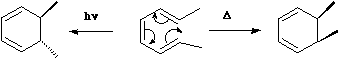
Cycloaddition and Cyclo-elimination reactions.
A cycloaddition reaction involves the
concerted formation of two or more sigma bonds between the termini of two or more conjugated ¼
systems. The reverse reaction involves the concerted cleavage of two or more sigma bonds to
produced two or more conjugated ¼ systems.
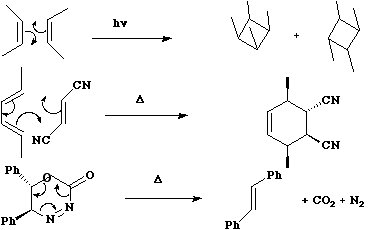
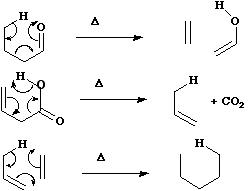
For the specific case where formation or cleavage of sigma bonds occurs at a single atom, the reaction is
called a Cheletropic reaction.
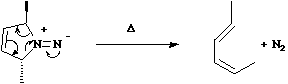
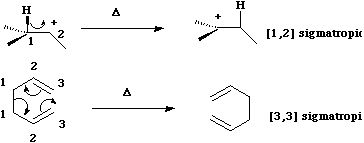
Sigmatropic Reactions.
A sigmatropic reaction involves the concerted migration of an atom or
group from one point of attachment to a conjugated system to another point of attachment, during
which one sigma bond is broken and another sigma bond is made. A sigmatropic reaction can be classified
according to the length of the group that migrates, and the length of the backbone along which it
migrates;
Ene Reactions.
A general category which involves the formation and cleavage of unequal
numbers of s bonds in a concerted cyclic transition state.
There are also 'odd' categories, normally involving cyclopropyl systems, which could be classified
under several of the categories described above.
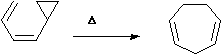
Return to index