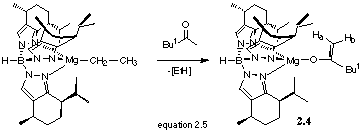
Reaction of TpmenthylMgCH2CH3 with ButCOMe
Addition of one equivalent of ButCOMe to 2.1 gave an off white solid that was identified as 2.4 by 1H NMR (equation 2.5).

Two singlets at d 4.31 and 3.86 ppm were assigned to two vinylidene protons, characteristic of an enolate derivative. IR data confirms the formation of an enolate. As well as nB-H at 2455 cm-1 (TpmenthylMgCH2CH3 gives nB-H at 2467cm-1), nC=C is observed at 1606 cm-1. 13C NMR also gave olefinic resonances at d 78.5 and 174.7 ppm. The data were similar to those obtained by Parkin et al[43] for TpButMg{h1-OC(=CH2)But} . The data show that the enolate 2.4 is formed.
Reaction of TpmenthylMgCH2CH3 with CyC(O)Me
The reaction of TpmenthylMgCH2CH3 (2.1) with 1 equivalent of CyC(O)Me afforded a white solid that was analysed by 1H NMR and IR (equation 2.6).
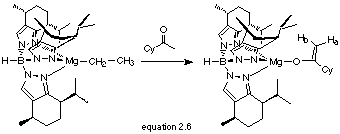
The 1H NMR showed a singlet at d 3.97 and 4.22 ppm, again indicative of enolate formation. The cyclohexyl protons were obscured by the menthylpyrazolyl protons and hence they could not be assigned. However, IR spectroscopy confirmed the formation of an enolate with nC=C at 1611 cm-1 and nB-H at 2458 cm-1. The limited solubility of the compound prevented a 13C NMR spectrum from being recorded.
Reaction of TpmenthylMgCH2CH3 with Acetone
The reaction of TpmenthylMgCH2CH3 (2.1) with one equivalent of acetone afforded a white solid in 81% yield (equation 2.7).
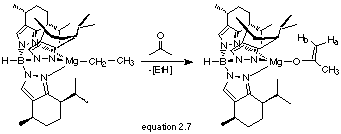
The product was only slightly soluble in C6D6, hence the 1H NMR was very weak and broad, but resonances at d 3.95 and 4.12 ppm support enolate formation as do IR absorptions nC=C at 1616 cm-1 and nB-H at 2457 cm-1.
Magnesium enolates can be isolated for sterically demanding ketones such
as ButC(O)Et [52] or (2,4,6-Me3C6H2)C(O)CH3 [53] and our observation of the formation
of an enolate with ButCOMe and CyC(O)Me is not surprising, but the formation
of an enolate with acetone demonstrates that the reactivity of the Mg-R
bond is not typical of Grignard reagents and appears to be unique to tris(pyrazolyl)hydroborato
magnesium alkyls.