| [Related articles/posters: None: require molecules] |
Montilla, Francisco; Pastor, Antonio; Galindo, Agustín*
Departamento de Química Inorgánica,
Aptdo 553, 41071 Sevilla, Spain
Imido ligands have been widely used as stabilizing ligands in high-oxidation-state transition metal complexes.1 Their chemistry has experienced a remarkable growth due to the role they play in many important reactions.
Following our interest in this area, we have extended
our recent results2 in the synthesis of
bis(imido) complexes of molybdenum, d0-Mo(NR)2,
to the related d0 organoimido complexes
of vanadium. In this contribution, we describe the synthesis and characterization
of several complexes of vanadium containing the imido ligand.
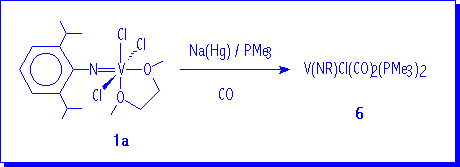
Treatment of VOCl3 with one equivalent of RNCO in refluxing octane led to V(NR)Cl3 compounds.3 The addition of 1,2-dimethoxyethane (dme) to solutions of V(NR)Cl3 produces the precipitation in a nearly quantitative yield of V(NR)Cl3(dme) (R = 2,6-iPr2C6H3, 1a; 1-adamantyl, 1b) as solid materials.
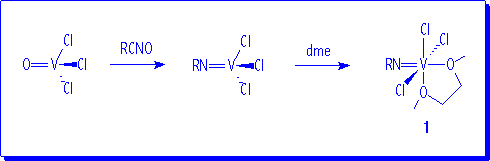
1H NMR and 13C{1H} NMR spectra of 1 are in agreement with this formulation and the proposed structure is similar to that recently reported for the analogous V(NtBu)Cl3(dme).4 Compounds 1 are employed as suitable starting materials for the synthesis of other imido complexes of vanadium.
Substitution reaction of 1a with Et2PCH2CH2PEt2 (depe) affords the expected complex V(N-2,6-iPr2C6H3)Cl3(depe), 2.
1H NMR and 13C{1H} NMR spectra of 2 show, besides the characteristic signals of the 2,6-iPr2C6H3 group, two set of resonances for the unequivalent P-Et and CH2-P groups. 31P{1H} NMR spectrum displays two broad resonances at 14.4 and 48.3 ppm. NMR data of 2 suggest a mer- distribution of the chloro atoms, similar to that found in the related2a organoimido molybdenum complex Mo(NR)Cl3(depe).
The reaction of 1a with monoanionic bidentate dithioligands (iPrOCS2- and iPr2NCS2-) gives V(N-2,6-iPr2C6H3)(S-S)3, (S-S = iPrOCS2, 3a; iPr2NCS2, 3b).
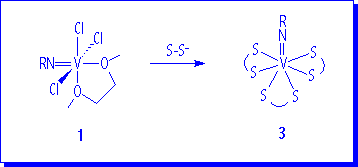
1H NMR spectra indicate that the complexes
are fluxional at room temperature (ca. 298 K). For 3b, all
three dithiocarbamate ligands produce a single pattern (CH3
broad signal at 1.25 ppm and CH very broad hump at 4.48 ppm), meanwhile
3a exhibits two separate absorptions (pseudotriplet + doublet, 2:1
ratio) for the methyl substituents of the xanthate ligands. For the latter
derivative the 1H NMR spectrum was recorded at 343
K, where the fast limit exchange was reached, and only one collection
of signals (doublet + heptet) was observed. At 303 K
the methyl groups become different and assuming the structure reported
for complex5a Nb(N-p-C6H4CH3)(S2CNEt2)3,
the two resonances can be ascribed to the different xanthate ligands occupying
the axial and equatorial positions. The fluxional NMR behavior of these
compounds is similar to that reported for the heavier group 5 metals, M(NR)(S2CNR'2)3.5
Interaction of 1 with one equivalent of the monoanionic tripod ligand6 LOEt (LOEt = (h-C5H5)Co{P(O)(OEt)2}3) affords the compounds (LOEt)V(NR)Cl2 (R = 2,6-iPr2C6H3, 4a; 1-adamantyl, 4b) as red crystalline materials in good yields.
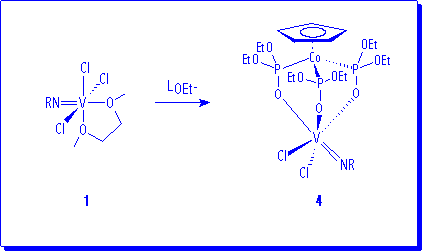
NMR spectra are in conformity with the proposed formulation. So, for example, three signals appear in the 1H NMR for the methyl groups, three resonances in the 13C{1H} NMR for the methylene groups of the LOEt ligand and an AX2 spin system arises in the 31P{1H} NMR spectrum. Related imido compounds of formulation (Cp)V(NR)Cl2 and (Tp)V(NR)Cl2 are known.7
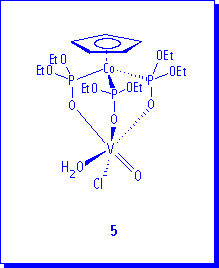
During the synthesis of 3a, a second product, 5, can be isolated from this reaction in the form of paramagnetic green crystals. A single crystal of 5 was analyzed by X-ray diffraction methods but unfortunately the refinement process was not possible to be completed. Nevertheless, the results confirm the formulation of 5 as compound (LOEt)V(=O)Cl(H2O). A similar vanadium complex, (LOEt)V(=O)(acac), was previously characterized by X-ray.8
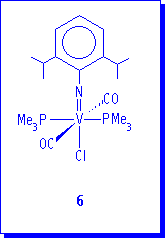
Finally, we like to present some preliminary results concerning the reduction of complex V(N-2,6-iPr2C6H3)Cl3(dme) to d2 organoimido vanadium derivatives. The sodium-amalgam reduction of 1a in the presence of two equivalents of PMe3 under atmosphere of CO produces a red solution from which is possible to isolate red crystals of compound 6.
6 is unstable in solution and even in the solid state under nitrogen, decomposing to an unidentified paramagnetic product through CO dissociation. The IR spectrum indicate the coordination of CO displaying a strong band at 1938 cm-1 (Nujol). The 31P{1H} NMR spectrum shows a broad plateau-form resonance for the phosphorous atoms at 0.8 ppm, caused by the unresolved coupling to the vanadium nucleus (I = 7/2) and quadrupolar relaxation effects. 1H and 13C{1H} NMR spectra of 6 are in agreement with two trans PMe3 ligands. The spectroscopic and analytical data agrees with the formulation V(N-2,6-iPr2C6H3)Cl(CO)2(PMe3)2 and a possible proposed structure for 6 is shown below. 6 represents, to our knowledge, the first free Cp vanadium(III) imido complex.
Acknowledgements
Financial support from MEC and Junta de Andalucia is gratefully acknowledged.
References
(1) (a) Wigley, D. E. Prog. Inorg. Chem. 1994, 42, 239. (b) Nugent, W. A.; Mayer, J. M. Metal-Ligand Multiple Bonds. Wiley Interscience: New York, 1988.
(2) Galindo, A.; Montilla, F.; Pastor, A.; Carmona, E.; Gutiérrez-Puebla, E.; Monge, A.; Ruiz, C. Inorg. Chem. 1997, 36, 2379.
(3) (a) Buijink, J.-K.; Teuben, J. H.; Kooijman, H.; Spek, A. L. Organometallics 1994, 13, 2922. (b) Devore, D. D.; Lichtenhan, J. D.; Takusagawa, F.; Maatta, E. A. J. Am. Chem. Soc. 1987, 109, 7408.
(4) Preuss, F.; Hornung, G.; Frank, W.; Rei, G.; Müller-Becker, S. Z. Anorg. Allg. Chem. 1995, 621, 1663.
(5) (a) Tan, L. S.; Goeden, G. V.; Haymore, B. L. Inorg. Chem. 1983, 22, 1744. (b) Nugent, W. A. Inorg. Chem. 1983, 22, 965.
(6) Kläui, W. Angew. Chem. Int. Ed. Engl. 1990, 29, 627.
(7) See for example: (a) Sundermeyer, J.; Putterlik, J.; Foth, M.; Field, J. S.; Ramesar, N. Chem. Ber. 1994, 127, 1201. (b) Preuss, F.; Wieland, T.; Günther, B. Z. Anorg. Allg. Chem. 1992, 609, 45. (c) Scheuer, S.; Fischer, J.; Kress, J. Organometallics 1995, 14, 2627.
(8) Román, E.; Tapia, F.; Barrera, M.; Garland, M. T.; Le Marouille, J.-Y.; Giannotti, C. J. Organomet. Chem. 1985, 297, C8.