Posts Tagged ‘X-ray’
Monday, April 1st, 2019
Members of the chemical FAIR data community have just met in Orlando (with help from the NSF, the American National Science Foundation) to discuss how such data is progressing in chemistry. There are a lot of themes converging at the moment. Thus this article[1] extolls the virtues of having raw NMR data available in natural product research, to which we added that such raw data should also be made FAIR (Findable, Accessible, Interoperable and Reusable) by virtue of adding rich metadata and then properly registering it so that it can be searched. These themes are combined in another article which made a recent appearance.[2]
(more…)
References
- J.B. McAlpine, S. Chen, A. Kutateladze, J.B. MacMillan, G. Appendino, A. Barison, M.A. Beniddir, M.W. Biavatti, S. Bluml, A. Boufridi, M.S. Butler, R.J. Capon, Y.H. Choi, D. Coppage, P. Crews, M.T. Crimmins, M. Csete, P. Dewapriya, J.M. Egan, M.J. Garson, G. Genta-Jouve, W.H. Gerwick, H. Gross, M.K. Harper, P. Hermanto, J.M. Hook, L. Hunter, D. Jeannerat, N. Ji, T.A. Johnson, D.G.I. Kingston, H. Koshino, H. Lee, G. Lewin, J. Li, R.G. Linington, M. Liu, K.L. McPhail, T.F. Molinski, B.S. Moore, J. Nam, R.P. Neupane, M. Niemitz, J. Nuzillard, N.H. Oberlies, F.M.M. Ocampos, G. Pan, R.J. Quinn, D.S. Reddy, J. Renault, J. Rivera-Chávez, W. Robien, C.M. Saunders, T.J. Schmidt, C. Seger, B. Shen, C. Steinbeck, H. Stuppner, S. Sturm, O. Taglialatela-Scafati, D.J. Tantillo, R. Verpoorte, B. Wang, C.M. Williams, P.G. Williams, J. Wist, J. Yue, C. Zhang, Z. Xu, C. Simmler, D.C. Lankin, J. Bisson, and G.F. Pauli, "The value of universally available raw NMR data for transparency, reproducibility, and integrity in natural product research", Natural Product Reports, vol. 36, pp. 35-107, 2019. https://doi.org/10.1039/c7np00064b
- A. Barba, S. Dominguez, C. Cobas, D.P. Martinsen, C. Romain, H.S. Rzepa, and F. Seoane, "Workflows Allowing Creation of Journal Article Supporting Information and Findable, Accessible, Interoperable, and Reusable (FAIR)-Enabled Publication of Spectroscopic Data", ACS Omega, vol. 4, pp. 3280-3286, 2019. https://doi.org/10.1021/acsomega.8b03005
Tags:American National Science Foundation, Bond length, ChemDraw, chemical, Chemistry, City: Orlando, Company: NSF, Force field, Intermolecular forces, Molecular geometry, National Science Foundation, natural product, Natural sciences, Orlando, Physical organic chemistry, Physical sciences, Quantum chemistry, Science and technology in the United States, Stereochemistry, steric energy, steric energy test, Strain, suitable free tool, unstable natural product, X-ray
Posted in Chemical IT | No Comments »
Friday, April 28th, 2017
Research data (and its management) is rapidly emerging as a focal point for the development of research dissemination practices. An important aspect of ensuring that such data remains fit for purpose is identifying what curation activities need to be associated with it. Here I revisit one particular case study associated with the molecular structure of a product identified from a photolysis reaction[1] and the curation of the crystallographic data associated with this study.
(more…)
References
- Y. Legrand, A. van der Lee, and M. Barboiu, "Single-Crystal X-ray Structure of 1,3-Dimethylcyclobutadiene by Confinement in a Crystalline Matrix", Science, vol. 329, pp. 299-302, 2010. https://doi.org/10.1126/science.1188002
Tags:assigned chemical name, author, chemical name, chemical name synonym, chemical names, chemical structures, editor, indicated chemical name synonym, Knowledge, radiation, Research, Scientific method, Technology/Internet, X-ray
Posted in Chemical IT, crystal_structure_mining | 5 Comments »
Monday, October 31st, 2016
Is asking a question such as “what is the smallest angle subtended at a chain of three connected 4-coordinate carbon atoms” just seeking another chemical record, or could it unearth interesting chemistry?
(more…)
Tags:animation, Bicyclic molecule, chemical record, Chemistry, City: Cambridge, Cycloalkane, Cyclopropanes, Java, Molecular geometry, Organic chemistry, potential energy surface, Safari, Web browser, X-ray
Posted in crystal_structure_mining, reaction mechanism | 7 Comments »
Saturday, April 2nd, 2016
A celebration of the life and work of the great chemist Paul von R. Schleyer was held this week in Erlangen, Germany. There were many fantastic talks given by some great chemists describing fascinating chemistry. Here I highlight the presentation given by Andy Streitwieser on the topic of organolithium chemistry, also a great interest of Schleyer's over the years. I single this talk out since I hope it illustrates why people still get together in person to talk about science.
(more…)
Tags:Centroid, chemical effect, chemical insights, chemical interpretation, City: Erlangen, Country: Germany, Degree of a continuous mapping, Ferrocene, Hydrogen bond, individual search definition, metal, overall search collection, Streitwieser, terminal H-positions, Torsion, X-ray
Posted in Chemical IT, crystal_structure_mining, Interesting chemistry | 6 Comments »
Tuesday, February 10th, 2015
Steganone is an unusual natural product, known for about 40 years now. The assignment of its absolute configurations makes for an interesting, on occasion rather confusing, and perhaps not entirely atypical story. I will start with the modern accepted stereochemical structure of this molecule, which comes in the form of two separately isolable atropisomers.

The first reported synthesis of this system in 1977 was racemic, and no stereochemistry is shown in the article (structure 2).[1] Three years later an “Asymmetric total synthesis of (-)steganone and revision of its absolute configuration” shows how the then accepted configuration (structure 1 in this article) needs to be revised to the enantiomer shown as structure 12 in the article[2] and matching the above representation. The system has continued to attract interest ever since[3],[4],[5],[6], not least because of the presence of axial chirality in the form of atropisomerism. Thus early on it was shown that the alternative atropisomer, the (aS,R,R) configuration initially emerges out of several syntheses, and has to be converted to the (aR,R,R) configuration by heating[3]. One could easily be fooled by such isomerism!
(more…)
References
- D. Becker, L.R. Hughes, and R.A. Raphael, "Total synthesis of the antileukaemic lignan (±)-steganacin", J. Chem. Soc., Perkin Trans. 1, pp. 1674-1681, 1977. https://doi.org/10.1039/p19770001674
- J. Robin, O. Gringore, and E. Brown, "Asymmetric total synthesis of the antileukaemic lignan precursor (-)steganone and revision of its absolute configuration", Tetrahedron Letters, vol. 21, pp. 2709-2712, 1980. https://doi.org/10.1016/s0040-4039(00)78586-8
- E.R. Larson, and R.A. Raphael, "Synthesis of (–)-steganone", J. Chem. Soc., Perkin Trans. 1, pp. 521-525, 1982. https://doi.org/10.1039/p19820000521
- A. Bradley, W.B. Motherwell, and F. Ujjainwalla, "A concise approach towards the synthesis of steganone analogues", Chemical Communications, pp. 917-918, 1999. https://doi.org/10.1039/a900743a
- M. Uemura, A. Daimon, and Y. Hayashi, "An asymmetric synthesis of an axially chiral biaryl via an (arene)chromium complex: formal synthesis of (–)-steganone", J. Chem. Soc., Chem. Commun., vol. 0, pp. 1943-1944, 1995. https://doi.org/10.1039/c39950001943
- B. Yalcouye, S. Choppin, A. Panossian, F.R. Leroux, and F. Colobert, "A Concise Atroposelective Formal Synthesis of (–)‐Steganone", European Journal of Organic Chemistry, vol. 2014, pp. 6285-6294, 2014. https://doi.org/10.1002/ejoc.201402761
Tags:free energy, lowest energy conformation, natural product, simulation, spectroscopy, unusual natural product, X-ray
Posted in Interesting chemistry | No Comments »
Saturday, July 5th, 2014
I was lucky enough to attend the announcement made in 2012 of the discovery of the Higgs Boson. It consisted of a hour-long talk mostly about statistics, and how the particle physics community can only claim a discovery when their data has achieved a 5σ confidence level. This represents a 1 in 3.5 million probability of the result occurring by chance. I started thinking: how much chemistry is asserted at that level of confidence? Today, I read Steve Bachrach’s post on the structure of Citrinalin B and how “use of Goodman’s DP4 method indicates a 100% probability that the structure of citrinalin B is (the structure below)”. Wow, that is even higher than the physicists. Of course, 100% has been obtained by rounding 99.7 (3σ is 99.73%) or whatever (this is one number that should never be so rounded!). 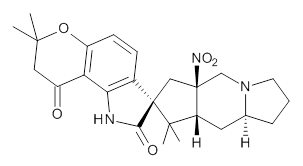 But there was one aspect of this that I did want to have a confidence level for; the absolute configuration of citrinalin B. Reading the article Steve quotes[1], one sees this aspect is attributed to ref 5[2], dating from 2005. There the configuration was assigned on the basis of “comparison of the electronic circular dichroism (ECD) spectra for 1 and 2 with those of known spirooxiindole alkaloids“. However, this method can fail[3]. Also, one finds “comparison of the vibrational circular dichroism (VCD) spectra of 1 with those of model compounds“[2]. Nowadays, one would say that there is no need for model compounds, why not measure and compute the VCD of the actual compound? Even a determination using the Flack crystallographic method can occasionally be wrong![4]. Which leads to asking what typical confidence levels might be for these three techniques, and indeed whether improving instrumentation means that the confidence level gets higher with time. OK, I am going to guess these.
But there was one aspect of this that I did want to have a confidence level for; the absolute configuration of citrinalin B. Reading the article Steve quotes[1], one sees this aspect is attributed to ref 5[2], dating from 2005. There the configuration was assigned on the basis of “comparison of the electronic circular dichroism (ECD) spectra for 1 and 2 with those of known spirooxiindole alkaloids“. However, this method can fail[3]. Also, one finds “comparison of the vibrational circular dichroism (VCD) spectra of 1 with those of model compounds“[2]. Nowadays, one would say that there is no need for model compounds, why not measure and compute the VCD of the actual compound? Even a determination using the Flack crystallographic method can occasionally be wrong![4]. Which leads to asking what typical confidence levels might be for these three techniques, and indeed whether improving instrumentation means that the confidence level gets higher with time. OK, I am going to guess these.
(more…)
References
- E.V. Mercado-Marin, P. Garcia-Reynaga, S. Romminger, E.F. Pimenta, D.K. Romney, M.W. Lodewyk, D.E. Williams, R.J. Andersen, S.J. Miller, D.J. Tantillo, R.G.S. Berlinck, and R. Sarpong, "Total synthesis and isolation of citrinalin and cyclopiamine congeners", Nature, vol. 509, pp. 318-324, 2014. https://doi.org/10.1038/nature13273
- T. Mugishima, M. Tsuda, Y. Kasai, H. Ishiyama, E. Fukushi, J. Kawabata, M. Watanabe, K. Akao, and J. Kobayashi, "Absolute Stereochemistry of Citrinadins A and B from Marine-Derived Fungus", The Journal of Organic Chemistry, vol. 70, pp. 9430-9435, 2005. https://doi.org/10.1021/jo051499o
- F. Cherblanc, Y. Lo, E. De Gussem, L. Alcazar‐Fuoli, E. Bignell, Y. He, N. Chapman‐Rothe, P. Bultinck, W.A. Herrebout, R. Brown, H.S. Rzepa, and M.J. Fuchter, "On the Determination of the Stereochemistry of Semisynthetic Natural Product Analogues using Chiroptical Spectroscopy: Desulfurization of Epidithiodioxopiperazine Fungal Metabolites", Chemistry – A European Journal, vol. 17, pp. 11868-11875, 2011. https://doi.org/10.1002/chem.201101129
- F.L. Cherblanc, Y. Lo, W.A. Herrebout, P. Bultinck, H.S. Rzepa, and M.J. Fuchter, "Mechanistic and Chiroptical Studies on the Desulfurization of Epidithiodioxopiperazines Reveal Universal Retention of Configuration at the Bridgehead Carbon Atoms", The Journal of Organic Chemistry, vol. 78, pp. 11646-11655, 2013. https://doi.org/10.1021/jo401316a
Tags:chemical, Reading, Steve Bachrach, X-ray
Posted in General | 2 Comments »
Wednesday, October 2nd, 2013
This is a continuation of the discussion started on Steve Bachrach’s blog about a molecule with a very short H…H interaction involving two Si-H groups with enforced proximity. It had been inferred from the X-ray structure[1] that the H…H distance was in the region of 1.50Å. It’s that cis-butene all over again! So is that H…H region a bond? Is it attractive or repulsive? Go read Steve’s blog first.
(more…)
References
- J. Zong, J.T. Mague, and R.A. Pascal, "Exceptional Steric Congestion in an <i>in</i>,<i>in</i>-Bis(hydrosilane)", Journal of the American Chemical Society, vol. 135, pp. 13235-13237, 2013. https://doi.org/10.1021/ja407398w
Tags:Postscript, Steve Bachrach, X-ray
Posted in Interesting chemistry | 17 Comments »
Wednesday, April 17th, 2013
This is another in the occasional series of “what a neat molecule”. In this case, more of a “what a neat idea”. The s-triazine below, when coordinated to eg ZnI2, forms what is called a metal-organic-framework, or MOF. A recent article[1] shows how such frameworks can be used to help solve a long-standing problem in structure determination, how to get a crystal structure on a compound that does not crystallise on its own.
(more…)
References
- Y. Inokuma, S. Yoshioka, J. Ariyoshi, T. Arai, Y. Hitora, K. Takada, S. Matsunaga, K. Rissanen, and M. Fujita, "X-ray analysis on the nanogram to microgram scale using porous complexes", Nature, vol. 495, pp. 461-466, 2013. https://doi.org/10.1038/nature11990
Tags:chair, marine natural product, metal, radiation, X-ray
Posted in Interesting chemistry | 2 Comments »
 But there was one aspect of this that I did want to have a confidence level for; the absolute configuration of citrinalin B. Reading the article Steve quotes
But there was one aspect of this that I did want to have a confidence level for; the absolute configuration of citrinalin B. Reading the article Steve quotes