Posts Tagged ‘HTML element’
Monday, May 29th, 2017
As the Internet and its Web-components age, so early pages start to decay as technology moves on. A few posts ago, I talked about the maintenance of a relatively simple page first hosted some 21 years ago. In my notes on the curation, I wrote the phrase “Less successful was the attempt to include buttons which could be used to annotate the structures with highlights. These buttons no longer work and will have to be entirely replaced in the future at some stage.” Well, that time has now come, for a rather more crucial page associated with a journal article published more recently in 2009.[1]
(more…)
References
- H.S. Rzepa, "Wormholes in chemical space connecting torus knot and torus link π-electron density topologies", Phys. Chem. Chem. Phys., vol. 11, pp. 1340-1345, 2009. https://doi.org/10.1039/b810301a
Tags:Applet, compression algorithm, computing, Cross-platform software, HTML, HTML element, Internet Journal, Java, Java applet, Java platform, jmol, Markup languages, Open formats, publishers site, publishers systems, technology moves, Technology/Internet, the Internet Journal, Web browser, web technologies, Web-components age, XML, XSLT
Posted in Chemical IT | 8 Comments »
Thursday, November 12th, 2015
Derek Lowe has a recent post entitled "Another Funny-Looking Structure Comes Through". He cites a recent medchem article[1] in which the following acetal sub-structure appears in a promising drug candidate (blue component below). His point is that orally taken drugs have to survive acid (green below) encountered in the stomach, and acetals are famously sensitive to hydrolysis (red below). But if X=NH2, compound "G-5555" is apparently stable to acids.[1] So I pose the question here; why?
(more…)
References
- C.O. Ndubaku, J.J. Crawford, J. Drobnick, I. Aliagas, D. Campbell, P. Dong, L.M. Dornan, S. Duron, J. Epler, L. Gazzard, C.E. Heise, K.P. Hoeflich, D. Jakubiak, H. La, W. Lee, B. Lin, J.P. Lyssikatos, J. Maksimoska, R. Marmorstein, L.J. Murray, T. O’Brien, A. Oh, S. Ramaswamy, W. Wang, X. Zhao, Y. Zhong, E. Blackwood, and J. Rudolph, "Design of Selective PAK1 Inhibitor G-5555: Improving Properties by Employing an Unorthodox Low-p
<i>K</i>
<sub>a</sub>
Polar Moiety", ACS Medicinal Chemistry Letters, vol. 6, pp. 1241-1246, 2015. https://doi.org/10.1021/acsmedchemlett.5b00398
Tags:Cambridge, Derek Lowe, HTML, HTML element, medchem, medical chemistry
Posted in reaction mechanism | 2 Comments »
Sunday, September 6th, 2015
An article entitled "Four Decades of the Chemistry of Planar Hypercoordinate Compounds"[1] was recently reviewed by Steve Bacharach on his blog, where you can also see comments. Given the recent crystallographic themes here, I thought I might try a search of the CSD (Cambridge structure database) to see whether anything interesting might emerge for tetracoordinate carbon.
(more…)
References
- L. Yang, E. Ganz, Z. Chen, Z. Wang, and P.V.R. Schleyer, "Four Decades of the Chemistry of Planar Hypercoordinate Compounds", Angewandte Chemie International Edition, vol. 54, pp. 9468-9501, 2015. https://doi.org/10.1002/anie.201410407
Tags:Angle, Cambridge, chemical bonding, Cycloalkane, Cyclopropane, HTML, HTML element, Ligand, metal, search definition, search results, search software, Steve Bacharach
Posted in Chemical IT, crystal_structure_mining | No Comments »
Tuesday, March 8th, 2011
The story of Monastral is not about a character in the Magic flute, but is a classic of chemical serendipity, collaboration between industry and university, theoretical influence, and of much else. Fortunately, much of that story is actually recorded on film (itself a unique archive dating from 1933 and being one of the very first colour films in existence!). Patrick Linstead, a young chemist then (he eventually rose to become rector of Imperial College) tells the story himself here. It is well worth watching, if only for its innocent social commentary on the English class system (and an attitude to laboratory safety that should not be copied nowadays). Here I will comment only on its colour and its aromaticity.
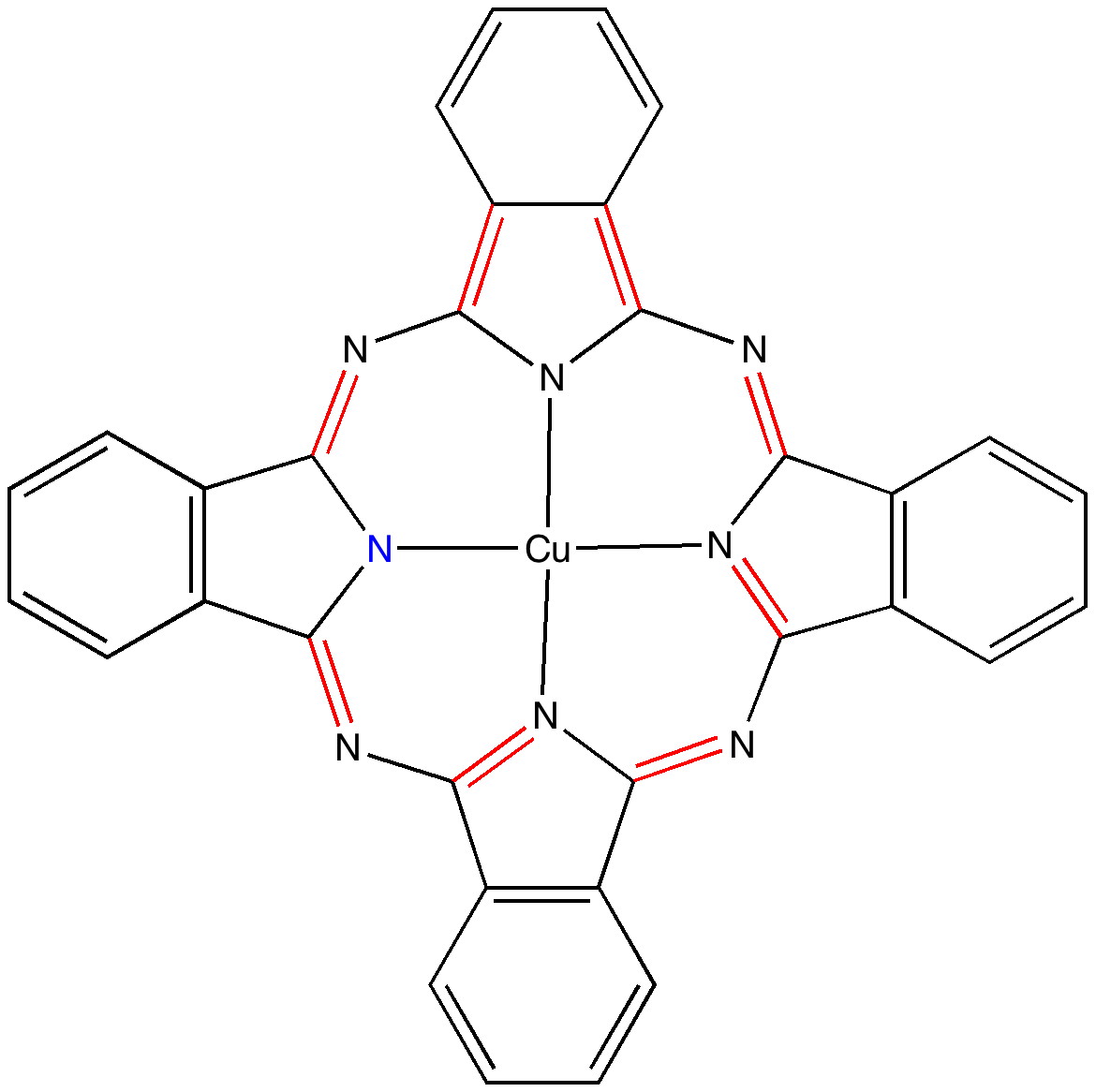
Copper phthalocyanine
(more…)
Tags:18 electron aromaticity, chemical serendipity, Historical, HTML, HTML element, Imperial College, Missouri, Monastral blue, Patrick Linstead, phthalocyanine, Phthalocyanine Blue BN, Phthalocyanines, Pigments, rector, young chemist
Posted in Interesting chemistry | 6 Comments »
