Posts Tagged ‘chair’
Wednesday, August 22nd, 2018
Following the general recognition of carbon as being tetrahedrally tetravalent in 1869 (Paterno) and 1874 (Van’t Hoff and Le Bell), an early seminal exploitation of this to the conformation of cyclohexane was by Hermann Sachse in 1890.[1] This was verified when the Braggs in 1913[2], followed by an oft-cited article by Mohr in 1918,[3] established the crystal structure of diamond as comprising repeating rings in the chair conformation.† So by 1926, you might imagine that the shape (or conformation as we would now call it) of cyclohexane would be well-known. No quite so for everyone!
(more…)
References
- H. Sachse, "Ueber die geometrischen Isomerien der Hexamethylenderivate", Berichte der deutschen chemischen Gesellschaft, vol. 23, pp. 1363-1370, 1890. https://doi.org/10.1002/cber.189002301216
- W.H. Bragg, and W.L. Bragg, "The structure of the diamond", Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character, vol. 89, pp. 277-291, 1913. https://doi.org/10.1098/rspa.1913.0084
- E. Mohr, "Die Baeyersche Spannungstheorie und die Struktur des Diamanten", Journal für Praktische Chemie, vol. 98, pp. 315-353, 1918. https://doi.org/10.1002/prac.19180980123
Tags:Carbon, chair, Chemistry, Conformation, Cycloalkanes, cyclohexane, Cyclohexane conformation, Derek Barton, Hermann Sachse, Hoff, Imperial College Chemical Society, Isomerism, Physical organic chemistry, R. F Hunter, Stereochemistry, Van 't Hof
Posted in Interesting chemistry | 1 Comment »
Sunday, December 7th, 2014
Continuing my hunt, here is a candidate for a strong(est?) halogen bond, this time between Se and I.[1]. 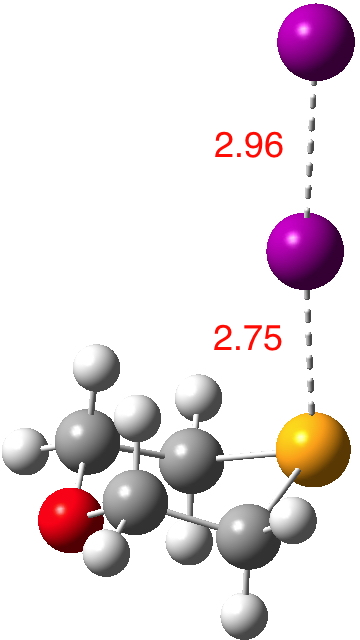 The features of interest include:
The features of interest include:
(more…)
References
- H. Maddox, and J.D. McCullough, "The Crystal and Molecular Structure of the Iodine Complex of 1-Oxa-4-selenacyclohexane, C<sub>4</sub>H<sub>8</sub>OSe.I<sub>2</sub>", Inorganic Chemistry, vol. 5, pp. 522-526, 1966. https://doi.org/10.1021/ic50038a006
Tags:chair, crystal structure search
Posted in crystal_structure_mining, Interesting chemistry | 7 Comments »
Sunday, April 20th, 2014
Ribulose-1,5-bisphosphate reacts with carbon dioxide to produce 3-keto-2-carboxyarabinitol 1,5-bisphosphate as the first step in the biochemical process of carbon fixation. It needs an enzyme to do this (Ribulose-1,5-bisphosphate carboxylase/oxygenase, or RuBisCO) and lots of ATP (adenosine triphosphate, produced by photosynthesis). Here I ask what the nature of the uncatalysed transition state is, and hence the task that might be facing the catalyst in reducing the activation barrier to that of a facile thermal reaction. I present my process in the order it was done‡.
(more…)
Tags:1M solutions, carbon fixation, chair, chemist, energy, free energy, low energy, low energy sink, lower energy conformation, lower energy isomer, Peter Medawar, phosphate
Posted in Interesting chemistry, reaction mechanism | 1 Comment »
Wednesday, April 17th, 2013
This is another in the occasional series of “what a neat molecule”. In this case, more of a “what a neat idea”. The s-triazine below, when coordinated to eg ZnI2, forms what is called a metal-organic-framework, or MOF. A recent article[1] shows how such frameworks can be used to help solve a long-standing problem in structure determination, how to get a crystal structure on a compound that does not crystallise on its own.
(more…)
References
- Y. Inokuma, S. Yoshioka, J. Ariyoshi, T. Arai, Y. Hitora, K. Takada, S. Matsunaga, K. Rissanen, and M. Fujita, "X-ray analysis on the nanogram to microgram scale using porous complexes", Nature, vol. 495, pp. 461-466, 2013. https://doi.org/10.1038/nature11990
Tags:chair, marine natural product, metal, radiation, X-ray
Posted in Interesting chemistry | 2 Comments »
Friday, May 13th, 2011
Conformational analysis comes from the classical renaissance of physical organic chemistry in the 1950s and 60s. The following problem is taken from E. D. Hughes and J. Wilby J. Chem. Soc., 1960, 4094-4101, DOI: 10.1039/JR9600004094, the essence of which is that Hofmann elimination of a neomenthyl derivative (C below) was observed as anomalously faster than its menthyl analogue. Of course, what is anomalous in one decade is a standard student problem (and one Nobel prize) five decades later.
(more…)
Tags:chair, conformational analysis, cyclic systems, energy, suitable molecular modelling software, Tutorial material
Posted in Uncategorised | 1 Comment »
Monday, April 4th, 2011
Most scientific theories emerge slowly, over decades, but others emerge fully formed virtually overnight as it were (think Einstein in 1905). A third category is the supernova type, burning brightly for a short while, but then vanishing (almost) without trace shortly thereafter. The structure of DNA (of which I have blogged elsewhere) belongs to the second class, whilst one of the brightest (and now entirely forgotten) examples of the supernova type concerns the structure of proteins. In 1936, it must have seemed a sure bet that the first person to come up with a successful theory of the origins of the (non-random) relatively rigid structure of proteins would inevitably win a Nobel prize. Of course this did happen for that other biologically important system, DNA, some 17 years later. Compelling structures for larger molecules providing reliable atom-atom distances based on crystallography were still in the future in 1936, and so structural theories contained a fair element of speculation and hopefully inspired guesswork (much as cosmological theories appear to have nowadays!).
(more…)
Tags:Cambridge, chair, Derek Barton, Dorothy Wrinch, energy, high energy species, Historical, mathematician, organic chemist, Patrick Coffey, relative free energy, thermodynamics, Tutorial material
Posted in Interesting chemistry | 2 Comments »
Sunday, May 2nd, 2010
Peter Murray-Rust in his blog asks for examples of the Scientific Semantic Web, a topic we have both been banging on about for ten years or more (DOI: 10.1021/ci000406v). What we are seeking of course is an example of how scientific connections have been made using inference logic from semantically rich statements to be found on the Web (ideally connections that might not have previously been spotted by humans, and lie overlooked and unloved in the scientific literature). Its a tough cookie, and I look forward to the examples that Peter identifies. Meanwhile, I thought I might share here a semantically rich molecule. OK, I identified this as such not by using the Web, but as someone who is in the process of delivering an undergraduate lecture course on the topic of conformational analysis. This course takes the form of presenting a set of rules or principles which relate to the conformations of molecules, and which themselves derive from quantum mechanics, and then illustrating them with selected annotated examples. To do this, a great many semantic connections have to be made, and in the current state of play, only a human can really hope to make most of these. We really look to the semantic web as it currently is to perhaps spot a few connections that might have been overlooked in this process. So, below is a molecule, and I have made a few semantic connections for it (but have not actually fully formalised them in this blog; that is a different topic I might return to at some time). I feel in my bones that more connections could be made, and offer the molecule here as the fuse!
(more…)
Tags:chair, chemical connections, Chemical IT, chemical world, chemist, energy, Fe, General, Interesting chemistry, lowest thermodynamic free energy, organic chemist, organometallic chemist, Peter Murray-Rust, semantic web, unusual
Posted in Chemical IT, General, Interesting chemistry | 2 Comments »
Thursday, January 28th, 2010
Like benzene, its fully saturated version cyclohexane represents an icon of organic chemistry. By 1890, the structure of planar benzene was pretty much understood, but organic chemistry was still struggling somewhat to fully embrace three rather than two dimensions. A grand-old-man of organic chemistry at the time, Adolf von Baeyer, believed that cyclohexane too was flat, and what he said went. So when a young upstart named Hermann Sachse suggested it was not flat, and furthermore could exist in two forms, which we now call chair and boat, no-one believed him. His was a trigonometric proof, deriving from the tetrahedral angle of 109.47 at carbon, and producing what he termed strainless rings.
(more…)
Tags:Adolf von Baeyer, animation, C, chair, conformational analysis, energy, General, Hermann Sachse, higher energy form, Interesting chemistry, Jonathan Goodman, minimum energy reaction path, model for cyclohexane, potential energy surface, symmetry breaking, Tutorial material
Posted in General, Interesting chemistry | 17 Comments »
