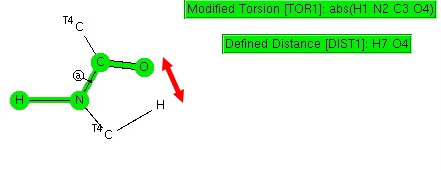
Amides with an H-N group are a component of the peptide linkage (O=C-NH). Here I ask what the conformation (it could also be called a configuration) about the C-N bond is. A search of the following type can be defined:
The dihedral shown is for H-N-C=O (but this is equivalent to the C-C-N-C dihedral, which is also often called the dihedral angle associated with the peptide group). I have also added a distance, from a C-H to the carbonyl oxygen. Other search constraints include T ≤ 175K, R < 0.05, no disorder, no errors, that neither N-C bonds are part of a ring and that the two carbons marked T4 both have four connected bonds. The search results in 619 hits (January 2013 version of the CCDC database), and these are displayed below.
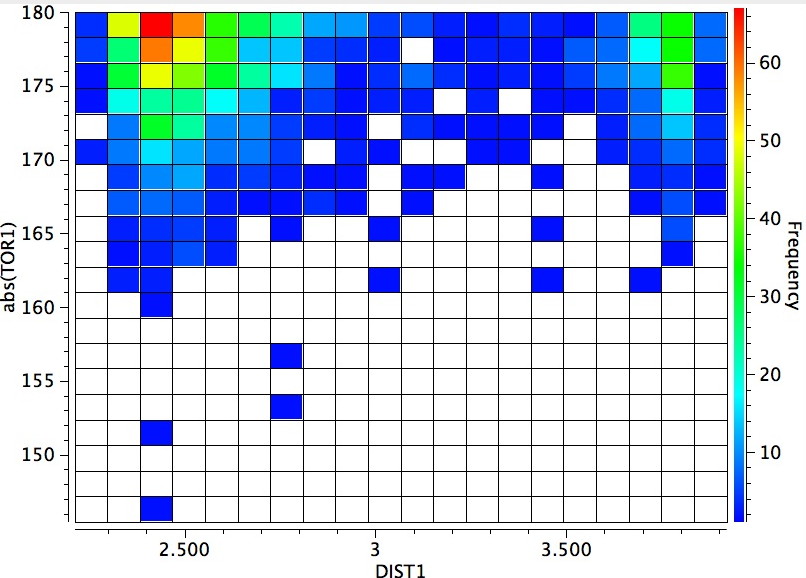
The horizontal axis reveals the highest concentration (red) at ~2.4Å due to a syn-co-planar alignment of the C-H bond with the plane of the C=O bond in the s-cis conformer (the significantly smaller hot-spot at ~3.9A may be due to an anti-co-planar alignment of this C-H bond).
The vertical axis shows a clear preference for a dihedral of 179° (in fact no hits with a dihedral of less than 14o° were found) and this can only arise from the s-cis conformation in which the H-N bond is oriented antiperiplanar to the axis of the C=O bond. This preference can be rationalised by filled/empty NBO-orbital interactions, which include:
- Antiperiplanar interaction between the N-H as donor and the C=O as a σ-acceptor (E(2) = 4.1 kcal/mol)
- Antiperiplanar interaction between the N-H as acceptor and C-H as donor (E(2) = 4.7 kcal/mol)
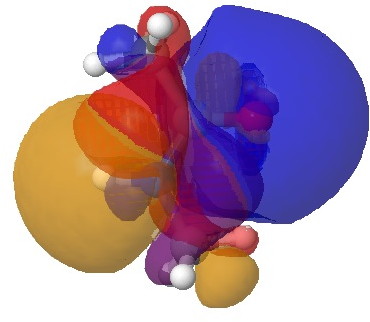
H-N/C=O. Click for 3D
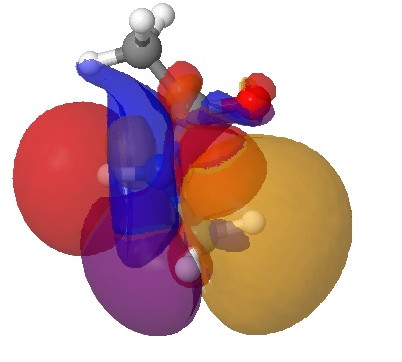
Click for 3D.
This latter overlap conspires to bring the C-H hydrogen close to the oxygen (~2.35Å, DIST1 in the diagram above). So one might be entitled to ask: is this a hydrogen bond? There are (at least) two ways of testing this.
- The NBO E(2) interaction energy between the oxygen in-plane lone pair and the H-C as acceptor is 0.8 kcal/mol. For hydrogen bonds, such E(2) energies more or less resemble the actual H-bond strengths, i.e. a strong H-bond has an E(2) energy of ~ 8 kcal/mol; and a medium O…H-C hydrogen bond weighs in at around 3 kcal/mol. So this one is very weak. This is due to poor overlap resulting from the small ring size (5).
- The NCI (non-covalent-interaction) surface does reveal a feature in the CH…O region, but the colour coding (which indicates how attractive/repulsive this is) is both pale blue (attractive) and yellow (repulsive). Again this is only consistent with a very weak overall H-bond.
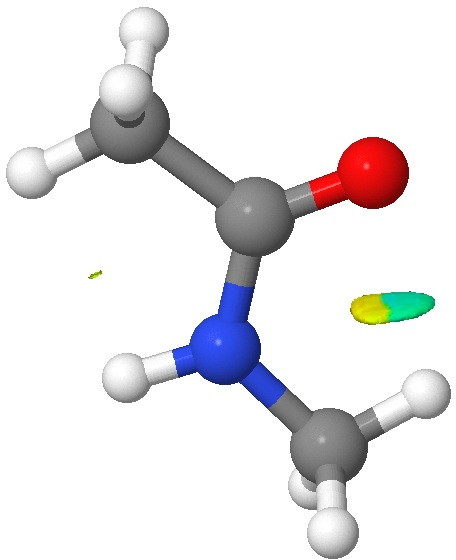
NCI surface. Click for 3D.
I end by reminding that the s-cis H-N-C=O conformation is a very common feature in peptides (the CCDC database comprises mostly small molecules, not larger peptides and proteins) arising from really quite subtle orbital interactions.
Acknowledgments
This post has been cross-posted in PDF format at Authorea.
Tags: conformational analysis, energy, interaction energy, Interesting chemistry, peptide, search constraints, search results, Tutorial material
[…] Chemistry with a twist « The conformational preference of s-cis amides. […]
[…] conclude my exploration of conformational preferences by taking a look at esters. As before, I start with a search definition, the ester being restricted to one bearing only sp3 carbon […]