The epoxidation of an alkene to give an oxirane is taught in introductory organic chemistry. Formulating an analogous mechanism for such reaction of an alkyne sounds straightforward, but one gradually realises that it requires raiding knowledge from several other areas of (perhaps slightly more advanced) chemistry to achieve a joined up approach to the problem. I had indeed hinted in a previous post that the mechanism for oxidation of acetylene to ketene might be an interesting arrow pushing challenge to set a bright tutorial group, and it was that self-hint that has led me to here. I now explore how my “arrow pushing” intuition stands up to a computational examination.
The products of the reaction of an acetylene with mCPBA (m-chloroperbenzoic acid) are set out[1] and the primary step of the mechanism[1] (this article declines to discuss the subsequent steps). The primary product is the formation of oxirene 1, a 4π-annulene which then undergoes an electrocyclic pericyclic ring opening to give the formyl carbene 4 (or 2 or 3). This presumed intermediate is also presumed to be highly reactive, and likely to undergo a variety of reactions (for example it will insert into a C-H bond if given one). I concentrate initially on just one path, the Wolff rearrangement (a [1,2] sigmatropic pericyclic reaction) to give the ketene 7. The scheme above constitutes one of those mandatory mechanistic challenges that organic chemists, almost without exception, cannot resist trying to solve, in the same way that some people may be addicted to Sudoko puzzles! So now for the reality check.
- The intrinsic reaction coordinate (IRC) for the reaction to form oxirene 1 is shown below (DOI: 10.14469/ch/10244). Its uneventful profile is deceptive.
- Look how delightfully non-linear the motions of the atoms are; one of the new C-O bonds clearly forms long before the second, in what is termed an asynchronous exothermic reaction. This is almost certainly due to the forming anti-aromaticity of the product, which tends to favour such asymmetry.
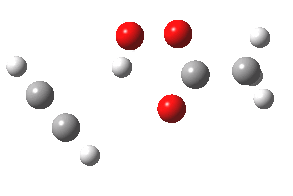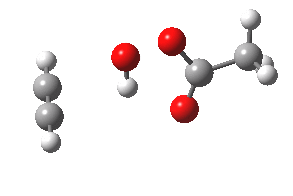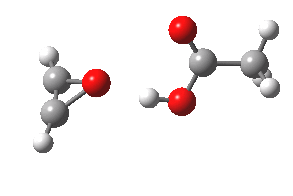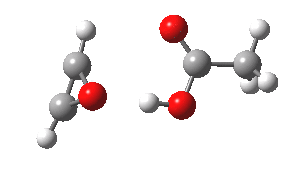
IRC for per oxidation of acetylene. Click for 3D
- Easily overlooked however would be the nature of the resulting product, which is actually a hydrogen-bonded complex of the oxirene and the acetic acid. Oxirene is a planar ring with two π-electrons from the double bond, and two πLp electrons from the oxygen. This makes it an anti-aromatic annulene, just like say cyclobutadiene. One aspect of this anti-aromaticity which is not often noted is that the anti-aromaticity makes these π electrons especially basic, and so it is with oxirene. The oxygen forms a remarkably short (1.673Å) hydrogen bond to the H-O part of the carboxylic acid (in the process slightly attenuating its otherwise unremitting anti-aromaticity).
- However, the IRC for the next stage is unexpected (DOI: 10.14469/ch/10245). According to the scheme above, the oxirene would ring open to give a carbene 3, at which point this species might be expected to steady itself before deciding what pathway it will undertake. Shown below is the route 1-3-6.
- Starting at IRC=-8, the oxirene encounters a blip of a barrier at IRC=-3.5, but the potential never actually settles into the carbene 3. Instead, it sails past it without much of a rest, and its momentum carries it onwards to perform a [1,2] hydrogen migration (TS@IRC=0.0), before finally settling into the unexpected carbene intermediate 6.
- Species 6 is no longer anti-aromatic, the oxygen is less basic, and the consequence is that the hydrogen bond to the adjacent H-O group lengthens to 1.998Å. This is a splendid lesson in how anti-aromaticity affects basicity, and not a lesson we had been expecting.
- What happens to 6? An electrocyclic ring opening occurs (DOI: 10.14469/ch/10246) with an odd abrupt start of the action at IRC=-4 and after the transition state, an exothermic descent to ketene 7.
- Note that the acetic acid fragment stays passive until late on, when it decides to re-form the hydrogen bond.
Well, that was a complicated story, and we have learnt a lot about (anti)aromaticity and hydrogen bonds in the process. There is one fly in the ointment however. If you look that the barriers that have to be overcome for the sequence 1-3-6-7 to occur, they are all quite large. In fact, the original report (for di-t-butyl acetylene[1]) does say very little ketene is actually formed. The next step would be to find lower energy pathways for reaction (such as possibly involving 2 or 4). But I will save that for a future posta future post.
References
- J. Ciabattoni, R.A. Campbell, C.A. Renner, and P.W. Concannon, "Peracid oxidation of acetylenes. 1,2-Methyl migration, cyclopropane formation, and stereoselective 1,5- and 1,6-transannular insertion", Journal of the American Chemical Society, vol. 92, pp. 3826-3828, 1970. http://dx.doi.org/10.1021/ja00715a068
Tags: anti-aromatic, ketene, lower energy pathways, pericyclic, Tutorial material
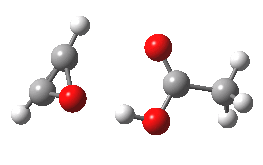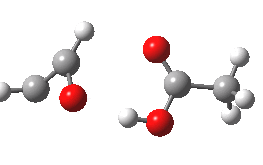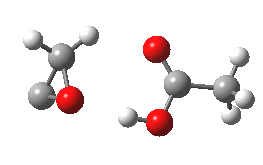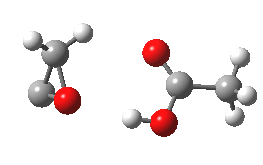
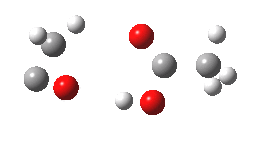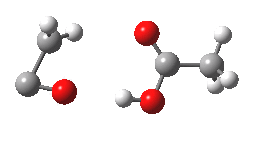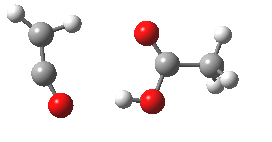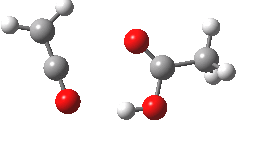
[…] next connection is to a post on how ethyne reacts with peracetic acid. The initial product of this reaction is oxirene, which like cyclobutadiene or cyclopropenium anion […]