An earlier post described how a (spherical) halide anion fitted snugly into a cavity generated by the simple molecule propanone, itself assembled by sodium cations coordinating to the oxygen. A recent elaboration of this theme, reminiscent of the children’s toys where objects have to be fitted into the only cavity that matches their shape, Nitschke and co-workers report the creation of a molecule with a tetrahedral rather than a spherical cavity (DOI: 10.1126/science.1175313 ), into which another but much smaller tetrahedral molecule is fitted. The small molecule is P4, in which each of the three valencies of the P atom is directed to a corner of the tetrahedron. The large molecule comprises four Fe atoms. These are each octahedrally coordinated with six ligand sites, three of which mimic the P atoms in also being directed towards the remaining three vertices of a tetrahedron.
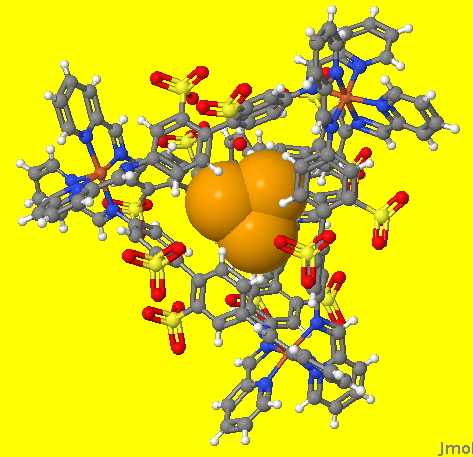
Needless to say, the properties of the P4 molecule when entrained into this larger container are nothing like that of the free molecule. Now it is quite inert, but this is due purely to the snug fit. For example, the normal reaction of this molecule is to oxidize in air. But such oxidation would now produce a molecule too big to fit into the cavity. Hence no reaction!
So, now the search is on for a cubic container to include a cubic molecule!
Tags: cavity, Interesting chemistry, pence, tetrahedral
[…] allows un-natural reactions to be undertaken. A recently famous example is the preparation of P4 (see blog post here), an otherwise highly reactive species which, when trapped in the cavity is now sufficiently […]