The 1H NMR spectrum of an aromatic molecule such as benzene is iconic; one learns that the unusual chemical shift of the protons (~δ 7-8 ppm) is due to their deshielding by a diatropic ring current resulting from the circulation of six aromatic π-electrons following the Hückel 4n+2 rule. But rather less well-known is the spectacular inversion of these effects as induced by the paratropic circulation of 4n electrons. A 4n+2 rule can be converted to a 4n one by the addition of two electrons, and chemically this can be done by reduction with lithium metal to form a dianion. Fortunately, this experiment has been done for a molecule known as methano[10]annulene. This is a 4n+2 aromatic molecule 1 with ten π-electrons (n=2) that can be reduced with lithium metal to form an ion-pair 2 comprising lithium cations and the twelve π-electron (4n, n=3) methano[10]annulene dianion.[1]
Here I ask whether these magnetic effects can be modelled using quantum mechanics. The point of interest here is the ion-pair. Can one get away with simply modelling the di-anion in say a continuum solvent (thf), or is the nearby presence of lithium cations (variously solvated) essential?
I used the model ωB97XD/TZVP/SCRF=THF (this DFT functional was shown to give good results for Rebek's encapsulated methane, itself manifesting lots of diamagnetic effects from the benzo groups of the capsule). Several models 3-6 for the di-anion were explored:
- A negatively charged species, stabilised by continuum solvation (thf).
- A neutral species, with two naked Li cations bound to the under face of the annulene
- Same as 4, but with one water molecule in the remaining coordination sphere of the Li.
- Same as 4, but with two MeOMe molecules in the remaining coordination sphere of the Li.
- Models 4-6 relate to what is called an intimate ion-pair (also a contact ion pair). We are not exploring the solvent-separated variety here.
I should also note that whereas the methane[10]annulene itself has C2v symmetry, the di-anion has the lower C2 symmetry, since the bond lengths are no longer approximately equal, a well known consequence of anti-aromaticity and associated paratropicity. Model 6 is shown below. It corresponds to two allyl anion.lithium cation ion pairs, separated by two localized double bonds.
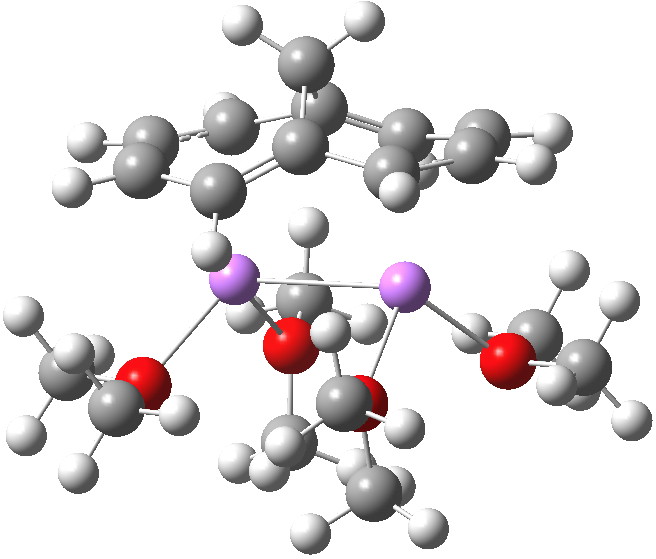
Click for 3D
The NMR for 1 and 2 and the models 3-6 is shown below. The shifts computed for the dianion are the average of two equilibrating forms, the barrier to which is fast on the NMR time scale. It would be fair to say that overall, the chemical shifts computed for the ion-pair model are a better fit to the recorded spectra than the purely anionic model. The most realistic ion-pair model, in which the lithium is also coordinated to two (dimethyl) ether oxygens, is a fair, if not perfect fit. Realistically in solution a number of dynamically equilibrating arrangements of the ion pair, possibly solvated by more ethers, or even to the extent of creating a solvent separated ion-pair, probably contribute to the overall Boltzmann populations.
| 1H | 13C | ||||||
|---|---|---|---|---|---|---|---|
| system/model | δ2,5,7,10 | δ3,4,8,9 | δ11 | δ2,5,7,10 | δ3,4,8,9 | δ1,6 | δ11 |
| 1[1] | 7.27 | 6.95 | -0.52 | 128.7 | 126.1 | 114.6 | 34.8 |
| 1(calc)[2] | 7.93 | 7.58 | -0.86 | 137.0 | 133.3 | 121.2 | 37.3 |
| 2 expt dianion[1] | 1.59 | 3.07 | 11.64 | 76.5 | 118.0 | 165.0 | 60.0 |
| 3(2–)[3] | 2.45 | 2.78 | 10.75 | 90.4 | 105.1 | 155.1 | 64.0 |
| 4(2–2Li+)[4] | 2.02 | 3.59 | 11.69 | 76.8 | 122.6 | 201.8 | 62.2 |
| 5(2–2Li+.H2O)[5] | 2.03 | 3.39 | 11.82 | 78.4 | 120.9 | 197.4 | 62.5 |
| 6(2–2Li+.2Me2O)[6] | 2.55 | 3.43 | 10.37 | 86.5 | 113.2 | 182.4 | 63.5 |
The most spectacular effect can be seen on the protons on C-11. For the neutral aromatic annulene, they are strongly shielded by a diatropic ring current. For the anti-aromatic di-anion, they are very strongly deshielded by a paratropic ring current, with Δδ 11-12 ppm. Such two-electron reductions (or oxidation) can yield equally spectacular effects on the NMR of other systems as well, as for example extended porphyrins.[7]
References
- D. Schmalz, and H. Günther, "1,6‐Methano[10]annulene Dianion, a Paratropic 12π‐Electron Dianion with a C10 Perimeter", Angewandte Chemie International Edition in English, vol. 27, pp. 1692-1693, 1988. http://dx.doi.org/10.1002/anie.198816921
- Henry S. Rzepa., "Gaussian Job Archive for C11H10", 2013. http://dx.doi.org/10.6084/m9.figshare.831450
- Henry S. Rzepa., "Gaussian Job Archive for C11H10(2-)", 2013. http://dx.doi.org/10.6084/m9.figshare.832421
- Henry S. Rzepa., "Gaussian Job Archive for C11H10Li2", 2013. http://dx.doi.org/10.6084/m9.figshare.832422
- Henry S. Rzepa., "Gaussian Job Archive for C11H14Li2O2", 2013. http://dx.doi.org/10.6084/m9.figshare.832423
- Henry S. Rzepa., "Gaussian Job Archive for C19H34Li2O4", 2013. http://dx.doi.org/10.6084/m9.figshare.832448
- C.S.M. Allan, and H.S. Rzepa, "Chiral Aromaticities. AIM and ELF Critical Point and NICS Magnetic Analyses of Möbius-Type Aromaticity and Homoaromaticity in Lemniscular Annulenes and Hexaphyrins", The Journal of Organic Chemistry, vol. 73, pp. 6615-6622, 2008. http://dx.doi.org/10.1021/jo801022b
Tags: chemical shifts, Interesting chemistry, lithium metal, unusual chemical shift